Strong electronic coupling of CoNi and N-doped-carbon for efficient urea-assisted H2 production at a large current density
Abstract
Exploiting efficient urea oxidation reaction (UOR) and hydrogen evolution reaction (HER) catalysts are significant for energy-saving H2 production through urea-assisted water electrolysis, but it is still challenging. Herein, carbon-encapsulated CoNi coupled with CoNiMoO (CoNi@CN-CoNiMoO) is prepared by solvothermal method and calcination to enhance the activity/stability of urea-assisted water electrolysis at large current density. It exhibits good activity for UOR (E10/1,000 = 1.29/1.40 V) and HER (E−10/−1000 = −45/−245 mV) in 1.0 M KOH + 0.5 M urea solution. For the UOR||HER system, CoNi@CN-CoNiMoO only needs 1.58 V at 500 mA cm−2 and shows good stability. Density functional theory calculation suggests that the strong electronic interaction at the interface between NiCo alloy and N-doping-carbon layers can optimize the adsorption/desorption energy of UOR/HER intermediates and accelerate the water dissociation, which can expedite urea decomposition and Volmer step, thus increasing the UOR and HER activity, respectively. This work provides a new solution to design UOR/HER catalysts for H2 production through urea-assisted water electrolysis.
1 INTRODUCTION
Replacing oxygen evolution reaction (OER) with urea oxidation reaction (UOR) is regarded as an effective strategy for energy-saving H2 generation because of the lower thermodynamic potential of UOR (0.37 V) than OER (1.23 V).1-3 Meanwhile, the electrocatalytic oxidation of urea can also purify the urea-rich sanitary/industrial wastewater to reduce its threats to the environment, and accelerate the nitrogen cycle process.4, 5 Unfortunately, UOR is a sluggish 6e− kinetics process [, reaction formulas 3–10 in Supporting Information,6, 7 which involves complicated intermediates transfer, CO-poisoning, and N2/CO2-desorption; therefore, it is necessary to develop high-performance catalysts for accelerating this process.8
Recently, researchers suggested that Ni-based materials can be applied as promising substitutes for noble metals because the self-oxidation behavior of Ni at ~1.35 V can form NiOOH active sites for UOR.2 The recognized UOR process on NiOOH surface is *+CO(NH2)2 → *CO(NH2)2 → *CO(NH2·NH) → *CO(NH·NH) → *CO(NH·N) → *CO(N2) → *CO(OH) + N2 → *CO(OH·OH) → *COO → * + CO2, in which * represents the active sites.6 Thus, the key intermediates are *CO(N2), *CO(NH·N), and *COO. However, due to the strong interaction between Ni3+ and *COO, as well as the sluggish oxidation of *CO(N2), the CO-poisoning and weak CO2-desorption usually occur in the UOR process for Ni-based materials.8, 9
To optimize the adsorption/desorption energies of UOR intermediates, Wang et al.10 discovered that the introduction of Co into β-Ni(OH)2 can effectively modulate its electron density distribution to form β-Co0.1Ni0.9-(OH)2 with hydrogen defect, hence improving UOR activity. Liu et al.9 proposed that the introduction of Co into Ni-based materials can improve the ability of anti-CO-poisoning for enhancing UOR activity. These results indicate that the combination of Ni and Co is effective to improving the UOR intrinsic activity. However, the reported catalysts cannot exhibit good performance for both HER and UOR, and the activity at large current densities needs to be further improved.
Constructing carbon-encapsulated metals and heterojunction have been regarded as effective methods to improve the UOR/HER performance,11-15 which can enhance the electron transfer and adjust the electronic structure to accelerate the reaction kinetics of UOR/HER.16-20 In addition, the carbon encapsulating layers can act as armor to keep the catalysts with high stability in harsh conditions.21, 22 Yang et al.23 designed the nitrogen-doped carbon nanotubes coated Ni with good UOR/HER activity. The authors suggested that24 nitrogen dopants are beneficial for CO2-desorption. Bao et al.22 demonstrated that carbon-encapsulating structure also can regulate the electronic structure of carbon/metal for the N-doped-carbon encapsulated CoNi alloy, thus enhancing the HER activity. Yang et al.25 designed the NiSe2/FeSe2 p-p heterojunction with good UOR/OER activity. The authors proposed that the charge redistribution at the interface between NiSe2 and FeSe2 favors the adsorption of urea and OH−, improving UOR/OER activity. But most of the carbon-encapsulated and heterojunction materials can only work at small current densities (<100 mA cm−2), and the UOR catalytic mechanism of these materials has rarely been clearly illustrated. Therefore, it is significant to prepare new catalytic materials with high performance for UOR/HER at large current densities (>500 mA cm−2), and study the intrinsic reasons for their high activity to meet the high criteria of commercial application.
This work combines the advantages of CoNi bimetals, carbon-encapsulating structure, heterojunction, and self-supporting strategy, and thus N-doped-carbon encapsulated CoNi combined with CoNiMoO (CoNi@CN-CoNiMoO) grown on nickel foam is prepared for high-efficiency urea-assisted H2 production. It only needs a low cell voltage of 1.58/1.67 V at 500/1000 mA cm−2 for the UOR||HER system, which is smaller than OER||HER system (1.88/2.01 V). Besides, it can maintain for 120 h at 500 mA cm−2 without significant performance decay, suggesting good stability at large current density. According to the density functional theory (DFT) results, the CoNi active sites are optimized by carbon-encapsulating structure to balance the adsorption/desorption energy of UOR intermediates for enhancing their activity. Furthermore, the strong electronic interaction between NiCo and N-doping-carbon layers is beneficial for water dissociation to accelerate the Volmer step, thus optimizing the H* Gibbs free energy (∆GH*) to boost HER activity.
2 RESULTS AND DISCUSSION
2.1 Physical characterization
The CoNi@CN-CoNiMoO is synthesized via two steps as shown in Figure 1A (Experimental details are displayed in Supporting Information). Figure S1 and Video 1 suggest that the nickel foam substrate has good mechanical stability. First, the nanorod precursor with smooth surface (Figure S2a,b) is prepared by the solvothermal method for 12 h at 160°C, which is composed of CoMo3O10∙4.6H2O and NiMo3O10∙9H2O (Figure S3). Then it is converted to CoNi@CN-CoNiMoO with nanorod/nanoparticles morphology (Figure S2c,d) by calcination at 350°C (N2 atmosphere, 2 h) and 450°C (H2/Ar atmosphere with volume ratio of 5%:95%, 2 h).
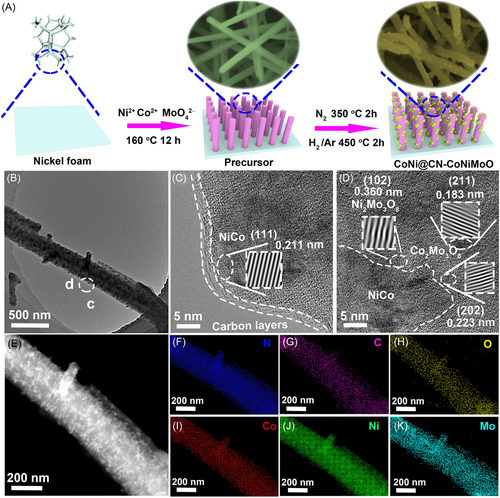
The composition and carbon defects of CoNi@CN-CoNiMoO are investigated by X-ray diffraction (XRD) and Raman spectrum. As illustrated in Figure S4, the XRD pattern suggests that the diffraction peaks are assigned to NiCo alloy [(111), (200), and (220) planes], Co2Mo3O8 (PDF#34-0511), and Ni2Mo3O8 (PDF#37-0855). According to the Raman spectrum (Figure S5), the area ratio of D (AreaD) to G (AreaG) is 1.29. This value is larger than that of the precursor (Figure S6), which is beneficial for UOR/HER.11, 23, 26 Furthermore, it is noticed that the calcination temperature in H2/Ar atmosphere is crucial for the formation of the ideal catalyst. As shown in SEM, Raman, and XRD results (Figures S1-9), NiCo alloy phase with lower crystallinity and carbon with fewer defects are obtained at lower temperatures, while the higher temperature leads to the destruction and shrinkage of nano morphology; therefore, 450°C is the optimum temperature to obtain the satisfactory structure. The transmission electron microscopy (TEM) image (Figure 1B) further shows the nanorod/nanoparticles morphology. The high-resolution TEM (HRTEM, Figure 1C) image indicates that the (111) plane of CoNi (0.211 nm) is encapsulated by approximately three carbon layers. The existence of carbon layers can not only activate the UOR/HER activity but also avoid corrosion of CoNi alloy by electrolyte for boosting the chemical stability.27, 28 Notably, the nanointerface can be clearly observed between CoNi alloy and CoNiMoO (Figure 1D), which is favorable for the UOR/HER activity.16 Besides, CoNiMoO is composed of Co2Mo3O8 [(202), (211)] and Ni2Mo3O8 [(102)], which is in accordance with the XRD result. Figure 1E–K indicates the uniform distribution of C, O, N, Co, Ni, and Mo elements.
X-ray photoelectron spectroscopy (XPS) is employed to further detect the chemical states of catalysts' surfaces. For CoNi@CN-CoNiMoO, the XPS survey spectrum (Figure S10) reveals the presence of C, O, N, Co, Ni, and Mo, which is in accordance with energy-dispersive X-ray spectroscopy mapping pictures. In the Ni 2p spectrum (Figure 2A), six peaks can be assigned to Ni0 2p (852.4/869.7 eV), Ni2+ 2p (854.9/872.2 eV), Ni3+ 2p (856.3/873.6 eV), and the other two peaks (861.6/878.9 eV) are ascribed to satellite peaks. In Figure 2B, the eight peaks located at 778.2/793.6, 782.8/798.2, 780.6/796, and 786.6/802 eV can be indexed to Co0 2p, Co2+ 2p, Co3+ 2p, and the satellite peak for Co 2p spectrum, respectively. For Mo 3d spectrum (Figure 2C), the peaks located at 232.1/229.0 (Mo4+ 3d), 232.9/229.8 (Mo5+ 3d), and 234.7/231.6 (Mo6+ 3d) eV are derived from Mo−O bonding.29 Because the sample is exposed to the air inevitably, the high valence Co, Ni, and Mo are detected.30, 31 For the O 1 s spectrum, two peaks at 532.3 and 530.8 eV are attributed to adsorbed-O and CoNiMo-O (Figure 2D), respectively. As displayed in Figure 2E, the C 1s of CoNi@CN-CoNiMoO are assigned to 284.8 (C−C), 286.5 (C−N), and 288.4 eV (C═O), and the pyridinic-N and graphitic-N are located at 398.4 and 401.4 eV (Figure 2F), respectively,32, 33 demonstrating the formation of N-doped-carbon.
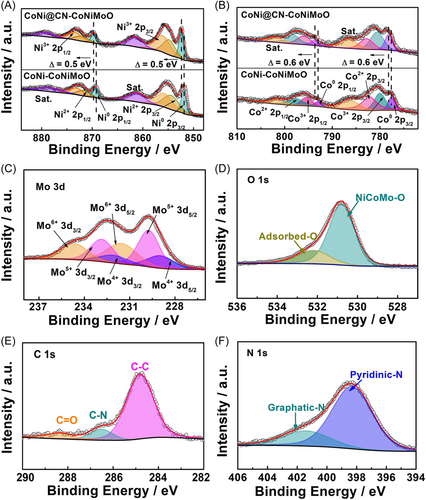
More importantly, the high-resolution XPS spectra of Co0 2p and Ni0 2p in CoNi@CN-CoNiMoO both exhibit positive shifts compared with CoNi-CoNiMoO (Figure 2A,B), implying the electronic interaction between N-doped-carbon and CoNi alloy. Furthermore, the charge density difference (Figure S45) of CoNi@CN-CoNiMoO indicates the electron transfer from CoNi alloy to N-doped-carbon at the interface. This interaction could obtain the positively charged CoNi core and negatively charged N-doped-carbon layers,28 which could act as active sites for UOR and HER, optimizing the adsorption/desorption energy of urea/hydrogen reaction intermediates and water dissociation energy to improve UOR/HER activity.
From the above results, the formation mechanism of CoNi@CN-CoNiMoO can be concluded as follows: (1) Ethylene glycol and H24Mo7N6O24∙4H2O are used as the carbon and nitrogen sources, respectively; (2) Due to the different enthalpy of Co, Ni, and Mo, parts of Co/Ni atoms will segregate from precursor to form the CoNi alloy; (3) The C and N sources can be in situ catalytically transformed into N-doped-carbon due to the catalytic effect of CoNi alloy22, 34, 35; (4) Mo and O atoms will combine with other Co and Ni atoms to form Co2Mo3O8 and Ni2Mo3O8. Therefore the heterostructure is formed between CoNi@CN and NiCoMoO.
2.2 Electrochemical characterization for UOR
The UOR activity of CoNi@CN-CoNiMoO is assessed by the standard three-electrode system in 1.0 M KOH with different concentrations of urea solution. Figure S11 shows that it has the best UOR activity in 1.0 M KOH with 0.5 M urea solution. Furthermore, the UOR linear sweep voltammetry (LSV) curves of CoNi@CN-CoNiMoO with/without iR correction are shown in Figure S12. And Figure 3A displays that the CoNi@CN-CoNiMoO with N-doped-carbon has faster UOR kinetics than CoNi-CoNiMoO.
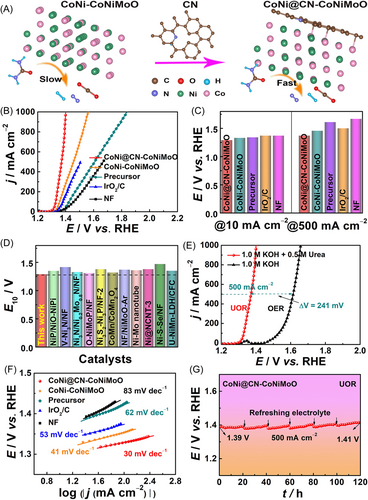
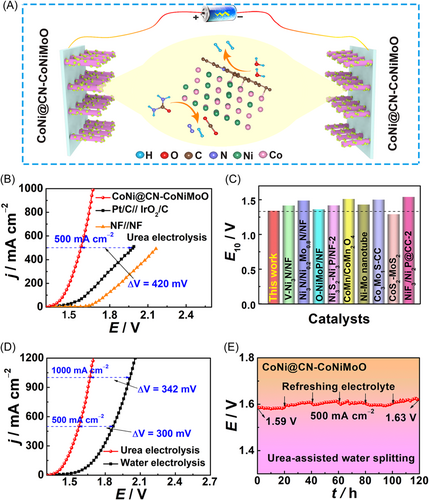
To meet the need for a large-scale application for UOR, catalysts must have low potentials at both small and large current densities. Figure 3B,C exhibits that CoNi@CN-CoNiMoO has low potentials of 1.29/1.37 V at 10/500 mA cm−2, which is superior to CoNi-CoNiMoO (E10/500 = 1.33/1.46 V), indicating that the N-doped-carbon encapsulating structure is beneficial for enhancing the UOR intrinsic activity. Besides, the UOR activity of CoNi@CN-CoNiMoO is also better than that of CoNi@CN (E10/500 = 1.32/1.49 V, Figure S13), which suggests that the formation of heterojunction composed of CoNiMoO and CoNi@CN can enhance the UOR activity. Meanwhile, the activity of CoNi@CN-CoNiMoO is significantly better than those of precursor (E10/500 = 1.34/1.61 V), IrO2/C (E10/500 = 1.35/1.50 V), NF (E10/500 = 1.37/1.67 V), and most of the reported Ni-based materials (Figure 3D and Table S1).6, 16, 23, 36-43 Importantly, CoNi@CN-CoNiMoO only needs 1.40 V at 1000 mA cm−2, which displays good prospect for industrial applications.
Meanwhile, Figure S14 shows that the best UOR activity is obtained by calcination at 450°C (H2/Ar atmosphere). CoNi@CN-CoNiMoO displays lower potentials than CoNi@CN-CoNiMoO-2 (E10/500 = 1.32/1.44 V) and CoNi@CN-CoNiMoO-3 (E10/500 = 1.31/1.42 V), indicating that the sample with a Ni:Co molar ratio of 1:1 has the highest UOR activity (Figure S15). Remarkably, CoNi@CN-CoNiMoO displays inferior OER activity (E500 = 1.61 V) (Figure 3E), which is larger than the potential (1.37 V) of UOR at 500 mA cm−2, suggesting that replacing the sluggish OER at the anode with a thermodynamically beneficial UOR is an efficient method to improve the energy conversion efficiency of water electrolysis.
Tafel slopes are acquired from LSV curves to evaluate the UOR kinetics of catalysts. CoNi@CN-CoNiMoO has a smaller Tafel slope (30 mV dec−1) than those of CoNi-CoNiMoO (41 mV dec−1), precursor (62 mV dec−1), IrO2/C (53 mV dec−1), and NF (83 mV dec−1), indicating that it has the fastest UOR kinetics among all catalysts (Figure 3F). Additionally, electrochemical impedance spectroscopy (EIS) is also employed to assess the UOR kinetics. Figure S16 illustrates that the CoNi@CN-CoNiMoO shows the lowest charge transfer resistance (Rct), which is consistent with Tafel results, confirming its good charge transfer kinetics in the UOR process. Subsequently, its UOR LSV curves at different scan rates (5~50 mV s−1) without iR correction in 1.0 M KOH + 0.5 M urea solution indicate the excellent charge transfer capability and non-capacitive-control characteristic of the catalyst during UOR (Figure S17).
Stability at large current density is an important index to evaluate its ability for practical application. Figure 3G shows the good UOR stability of CoNi@CN-CoNiMoO at 500 mA cm−2 for 120 h in 1.0 M KOH + 0.5 M urea solution, which is better than those of CoNi-CoNiMoO (Figure S18) and CoNi@CN (Figure S19). This result further verifies that the N-doped-carbon encapsulating structure and the existence of heterojunction can effectively improve the stability of the catalyst, because the N-doped-carbon can act as armor to avoid the corrosion of electrolytes toward the internal metal. Furthermore, the heterojunction structure composed of CoNiMoO and CoNi@CN presents the nanorod/nanoparticles morphology supported on nickel foam. This morphology can expose more active sites and prevent the aggregation and Ostwald ripening of the catalyst to improve stability. Meanwhile, it can also facilitate the gas and liquid transfer during the urea-assisted water electrolysis process at a large current density.
Additionally, Figure S20 shows the negligible changes for LSV and Nyquist curves of CoNi@CN-CoNiMoO before and after the chronopotentiometry (CP) test. Additionally, the SEM (Figure S21), TEM (Figure S22), and Raman (Figure S23) results after the CP test all indicate its good stability. For XPS results (Figure S24a,b), the Ni0 and Co0 of CoNi@CN-CoNiMoO disappear after the UOR stability test, which could be due to the formation of NiOOH/CoOOH active sites (Supporting Information, Reaction formulas 11–16),44-46 thus maintaining high UOR activity at large current density during the stability test.
In Figure S24c, Mo4+ is oxidized to Mo6+ and Mo5+, which can conduce to the conversion of Ni2+/Co2+ into Ni3+/Co3+ to generate NiOOH/CoOOH for boosting UOR activity. Besides, the combination of Mo, Ni, and Co could optimize the adsorption/desorption energy of *NH2 and *CO intermediates on the active sites for UOR.47 The existence of Mo can make the Co/Ni element easier to be oxidized to a higher oxidation state and promote the oxidation of *CO, thereby reducing UOR overpotential and improving stability.48 Meanwhile, the presence of Co atoms and N-doping-carbon layers can promote the oxidation of carbonaceous compounds to enhance the ability of anti-CO/CO2 poisoning for improving UOR durability.9, 23
2.3 Electrochemical characterization for HER
The HER performance of CoNi@CN-CoNiMoO is investigated in 1.0 M KOH + 0.5 M urea solution. Figure 4A indicates that it shows better HER kinetics than CoNi-CoNiMoO. Figure S25 displays the HER LSV curves of the catalyst with/without iR correction. In Figure 4B,C, it is worth noting that CoNi@CN-CoNiMoO (E−10/−500 = −45/−168 mV) shows higher HER activity than CoNi-CoNiMoO (E−10/−500 = −60/−284 mV). This result suggests that the N-doped-carbon structure can effectively improve the HER intrinsic activity at both small and large current densities. The HER activity of CoNi@CN-CoNiMoO surpasses that of CoNi@CN (E−10/−500 = −85/−362 mV, Figure S26), indicating that the heterojunction composed of CoNiMoO and CoNi@CN is beneficial for enhancing the HER intrinsic activity. In addition, the HER activity of CoNi@CN-CoNiMoO is better than those of precursor (E−10/−500 = −148/−431 mV) and NF (E−10/−500 = −229/−539 mV), as well as most of the reported Ni-based materials (Figure 4D and Table S2),6, 16, 23, 36-39, 41 and it is close to Pt/C (E−10/−500 = −26/−157 mV). Notably, it only needs −245 mV at −1000 mA cm−2, exhibiting the prospect of industrial applications.
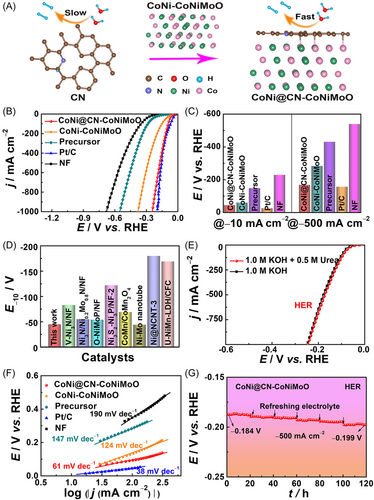
Figure 4E implies that the existence of urea in 1.0 M KOH electrolyte has no obvious effect on HER activity. And the best HER activity is obtained by calcination at 450°C in H2/Ar atmosphere (Figure S27). Furthermore, CoNi@CN-CoNiMoO needs lower potentials to drive −10/500 mA cm−2 than CoNi@CN-CoNiMoO-2 (E−10/−500 = −69/−258 mV) and CoNi@CN-CoNiMoO-3 (E−10/−500 = −58/−246 mV, Figure S28), suggesting that 1:1 is the optimal molar ratio of Ni to Co for HER activity.
Subsequently, the HER kinetics of CoNi@CN-CoNiMoO are researched by Tafel slopes and EIS. Its Tafel slope (Figure 4F, 61 mV dec−1) is much smaller than those of CoNi-CoNiMoO (124 mV dec−1), precursor (147 mV dec−1), and NF (190 mV dec−1), and close to Pt/C (38 mV dec−1), implying that it follows the Vomer–Heyrovsky mechanism.27, 49 Furthermore, CoNi@CN-CoNiMoO shows the lowest Rct among all catalysts. The above results indicate its fast electron transfer rate and remarkable HER kinetics. Figure S29 implies that it has excellent charge transfer capability. And it undergoes noncapacitive control for HER (Figure S30). Meanwhile, its Faraday efficiency is close to 100% (Figure S31), which is derived from the volume of H2 theoretically calculated and experimentally measured. Electrochemical surface area (ECSA) is proportional to double-layer capacitance (Cdl) measured by the cyclic voltammetry (CV) method (Figure S32a,c,e), which can reflect the number of active sites. The Cdl value of CoNi@CN-CoNiMoO (145.13 mF) is superior to CoNi-CoNiMoO (71.67 mF) and CoNi@CN (35.50 mF) (Figure S32b,d,f), indicating that it possesses the most active sites among all the samples. And the UOR and HER LSV curves are normalized by ECSA (Figure S33),50 which is in accordance with the initial results, implying that the N-doped-carbon encapsulating structure and heterojunction of CoNi@CN and CoNiMoO are beneficial for increasing the number of active sites and boosting UOR/HER intrinsic activity.
CoNi@CN-CoNiMoO also shows better HER stability at −500 mA cm−2 (Figure 4G) than CoNi-CoNiMoO (Figure S34) and CoNi@CN (Figure S35), suggesting that the stability of the catalyst can be enhanced by the N-doped-carbon encapsulating structure and the heterojunction. The reason for such good HER stability is the same as UOR. Besides, Figure S36 shows that the changes in LSV and Nyquist curves before and after the CP test are negligible. SEM (Figure S37), TEM (Figure S38), Raman (Figure S39), and XPS (Figure S40) results after the CP test all indicate good HER stability.
2.4 Urea-assisted water electrolysis
Considering the good UOR/HER activity of CoNi@CN-CoNiMoO, it is used as the cathode and anode simultaneously for urea-assisted water electrolysis (Figure 5A). It only needs 1.34/1.58 V to reach 10/500 mA cm−2 (Figure 5B), which is obviously smaller than those of the Pt/C//IrO2/C system (E10/500 = 1.40/1.99 V) and most of the reported values (Figure 5C and Table S3),6, 16, 17, 36, 38, 39, 41, 51, 52 confirming its prospect for urea-assisted water electrolysis. CoNi@CN-CoNiMoO only requires voltages of 1.34/1.58/1.67 V at 10/500/1,000 mA cm−2 for the UOR||HER system (Figure 5D), which is 70/300/342 mV lower than that of the OER||HER system (1.41/1.88/2.01 V), verifying that urea-assisted water electrolysis is energy-efficient for H2 generation. Additionally, the UOR||HER system can continuously produce H2 for 120 h at 500 mA cm−2 (Figure 5E), showing its potential for large-scale H2 generation and urea-rich wastewater purification.
2.5 DFT calculation for UOR and HER
DFT calculations are employed to verify the synergistic effect between CoNi alloy and N-doped-carbon, which can enhance the UOR/HER performance of the catalyst. Combined with the HRTEM/XPS/XRD results, theoretical calculation, and works of literature reported,26 the models of N-doped-carbon, CoNi-CoNiMoO, and CoNi@CN-CoNiMoO with different molar ratios of Ni/Co are constructed (Figure S41). Generally, the adsorption energy of water and urea molecules on the surface of the catalyst is a crucial index in the process of urea oxidation, because UOR and OER will compete with each other.53 Models for water and urea adsorption on different catalysts are displayed in Figures S42 and S43. Figure S44 suggests that the urea molecule is more easily adsorbed on the catalyst than on the water molecule, indicating the dominance of UOR over OER. More importantly, CoNi@CN-CoNiMoO (−1.16 eV) exhibits stronger urea adsorption energy compared with CoNi-CoNiMoO (−1.12 eV), which indicates that the N-doped-carbon encapsulating structure can effectively enhance the urea adsorption, facilitating UOR process. Besides, the urea molecule adsorption on the surface of CoNi alloy with different ratios of Ni/Co before/after N-doped-carbon encapsulation is an exothermic reaction; therefore, the urea molecule adsorption is a spontaneous reaction on the active site. The urea adsorption energy on CoNi@CN-CoNiMoO (−1.16 eV) is moderate, which is stronger than those of CoNi@CN-CoNiMoO-2 (−1.08 eV) and weaker than CoNi@CN-CoNiMoO-3 (−1.25 eV), suggesting that the molar ratio of Ni/Co plays an important role in regulating the adsorption energy of urea molecule.
The free energy of the UOR pathway is calculated to reveal the superiority of CoNi@CN-CoNiMoO. *CON2 is formed firstly by dehydrogenation of the urea molecules adsorbed on the surface of catalysts [*CO(NH2·NH2) → *CO(NH·NH2) → *CO(N·NH2) → *CO(NH·N) → *CON2], and the generated ∆G value of CoNi@CN-CoNiMoO is smaller than that of CoNi-CoNiMoO, especially for the rate-determining step (RDS, Figure 6A). This result can be ascribed to that the N-doping-carbon encapsulating structure can effectively reduce the electron density of Co/Ni, as shown in the charge density difference plot (Figure S45), which can facilitate the cleavage of N−H bonds. Besides, during this 6e− reaction (Figure 6A), Co atoms with high oxygen affinity can stabilize the carbonyl group, while the highly active Ni atoms combine with the N atoms to enhance the UOR kinetics. Then, *CON2 undergoes C−N breakage and N−N coupling processes to form *CO. Subsequently, the *CO is attacked by OH− to generate *COOH. Due to the existence of N-doping-carbon and the catalytic oxidation ability of Co to carbon monoxide,9 CoNi@CN-CoNiMoO obtains a lower ∆G value of *COOH than CoNi-CoNiMoO, and this can effectively alleviate poisoning on the catalyst. Furthermore, compared with traditional nickel-based materials, the RDS (Figure 6A) changes from *COO → * + CO2 to *CO(NH2)2 → *CO(NH·NH2) on both the surface of CoNi@CN-CoNiMoO and CoNi-CoNiMoO and the CO2-desorption is a spontaneous reaction, which can improve the anti-poisoning ability for CO2.23 Partial density of states (PDOS) are employed to further study the essential reasons for the enhanced activity. In Figure 6B, the Ni PDOS of CoNi@CN-CoNiMoO shows a value (−0.95 eV) closer to Ef, which acts as the active site for fast electron transfer. Meanwhile, C and N orbitals show a wide distribution range located at the deep positions of the Ef, suggesting the electron-rich characteristic, which can not only contribute to maintaining the valence of Ni/Co site but also balance the adsorption/desorption of reaction intermediates on the active sites. More importantly, compared with CoNi-CoNiMoO, the d-band centers of Co and Ni for CoNi@CN-CoNiMoO (Figure 6B) shift up. These results indicate that N-doping-carbon layers can modulate the electronic structure of Ni/Co, accelerating electron transfer to enhance the UOR activity. Besides, the Ni d-band center of CoNi@CN-CoNiMoO-2 and CoNi@CN-CoNiMoO-3 exhibits larger or smaller shifts than that of CoNi@CN-CoNiMoO in Figure 6B, which results in the strong or weak adsorption for UOR intermediates. This result reveals that CoNi@CN-CoNiMoO has optimum adsorption for UOR intermediates in comparison to CoNi@CN-CoNiMoO-2 and CoNi@CN-CoNiMoO-3, showing the highest UOR intrinsic activity.
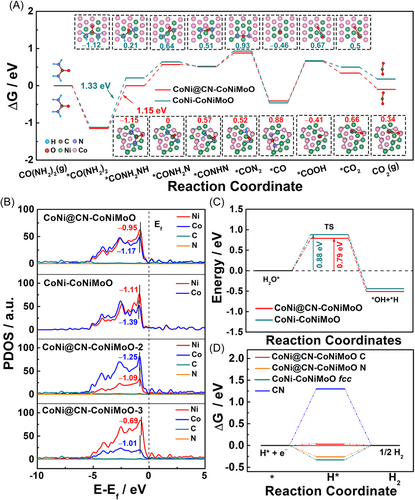
Generally, the ortho-carbon of pyridine-N acts as the HER active site;26, 54 thus, it is used as a research object in this work, and the *H adsorption sites of CN, CoNi-CoNiMoO, CoNi@CN-CoNiMoO, CoNi@CN-CoNiMoO-2, and CoNi@CN-CoNiMoO-3 are shown in Figures S46 and S47. As known, due to the lack of hydrogen ions in alkaline solutions, the *H is formed only by water dissociation;55-57 and thus, the Volmer step is important for HER. In Figure 6C, CoNi@CN-CoNiMoO (0.79 eV) displays a lower water dissociation energy than CoNi-CoNiMoO (0.88 eV), indicating that the former with N-doping-carbon layers can accelerate the Volmer step. Besides, the H* Gibbs free energy value (∆GH*) is an important HER activity descriptor.58, 59 It can be seen in Figure 6D that the ∆GH* value (0.03 eV) closer to zero can be obtained for the ortho-carbon of CoNi@CN-CoNiMoO in comparison with that of N-doped-carbon (1.3 eV) and CoNi-CoNiMoO (fcc, −0.33 eV), indicating that the N-doped-carbon encapsulating structure can accelerate the HER kinetics. As Figure S48 indicated, the ∆GH* value for the ortho-carbon of CoNi@CN-CoNiMoO models is also closer to zero than those of CoNi@CN-CoNiMoO-2 (0.41 eV) and CoNi@CN-CoNiMoO-3 (−0.29 eV), which implies that the Ni/Co ratios have an influence on the HER activity of N-doped-carbon.
Simultaneously, the PDOS and COHP are used to study the electronic structure of the N-doped-carbon and the interaction strength between ortho-carbon and hydrogen, respectively. In Figure S49a, the PDOS of C in CoNi@CN-CoNiMoO exhibits a broad range compared to N-doped-carbon. This result indicates that the PDOS with a broad range is beneficial for stabilizing the bond between the active site and hydrogen to optimize the adsorption of hydrogen and boost the charge-transfer kinetics of HER. Besides, the data for Ni/Co ratio also shows similar results (Figure S49b). Meanwhile, in Figure S50, the COHP of C−H for CoNi@CN-CoNiMoO (1.66) is larger than that of N-doped-carbon (1.60), suggesting that the stronger C−H bonding can be obtained on the surface of carbon for CoNi@CN-CoNiMoO. Besides, as shown in Figure S51, it displays the moderate COHP value (derived from PDOS, Figure S52) of C−H compared to CoNi@CN-CoNiMoO-2 (1.72) and CoNi@CN-CoNiMoO-3 (1.64), which indicates that CoNi@CN-CoNiMoO has the optimal adsorption/desorption for hydrogen intermediates. The above results show that the N-doped-carbon encapsulated CoNi alloy structure can effectively adjust the electronic structure of both N-doped-carbon and CoNi to optimize the adsorption/desorption of the urea/hydrogen reaction intermediates, thus increasing the UOR/HER intrinsic activity.
3 CONCLUSIONS
In conclusion, the N-doped-carbon encapsulated CoNi coupled with CoNiMoO grown on nickel foam is prepared for high-efficiency urea-assisted H2 production at large current density. It requires low potentials for UOR (E10/1000 = 1.29/1.40 V) and HER (E−10/−1000 = −45/−245 mV) and displays the satisfied stability toward UOR/HER at 500 mA cm−2 in 1.0 M KOH + 0.5 M urea solution. Importantly, for UOR||HER system, it displays a low cell voltage of 1.58/1.67 V at 500/1000 mA cm−2, which is 300/342 mV less than that of the OER||HER system. Furthermore, it also exhibits superior stability at 500 mA cm−2 for 120 h, implying the potential for commercial applications. DFT calculation indicates that it can not only balance the adsorption/desorption energy of urea/hydrogen reaction intermediates on the active sites for UOR/HER but also reduce the water dissociation energy to accelerate the Volmer step, enhancing HER activity. Hence, this work provides an effective strategy to design high-performance catalysts for energy-saving H2 production by urea-assisted water electrolysis.
ACKNOWLEDGMENTS
This work is supported by the National Natural Science Foundation of China (22162004), the Excellent Scholars and Innovation Team of Guangxi Universities, the Innovation Project of Guangxi Graduate Education (YCBZ2022038), and the High-performance Computing Platform of Guangxi University.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.




