Bifunctional PdPt bimetallenes for formate oxidation-boosted water electrolysis
Abstract
Small-molecule electrooxidation-boosted water electrolysis (WE) is an energy-saving method for hydrogen (H2) production. Herein, PdPt bimetallenes (PdPt BMLs) are obtained through the simple galvanic replacement reaction. PdPt BMLs reveal 2.93-fold enhancement in intrinsic electroactivity and 4.53-fold enhancement in mass electroactivity for the formate oxidation reaction (FOR) with respect to Pd metallenes (Pd MLs) at 0.50 V potential due to the synergistic effect. Meanwhile, the introduction of Pt atoms also considerably increases the electroactivity of PdPt BMLs for hydrogen evolution reaction (HER) with respect to Pd MLs in an alkaline medium, which even exceeds that with the use of commercial Pt nanocrystals. Inspired by the outstanding FOR and HER electroactivity of bifunctional PdPt BMLs, a two-electrode FOR-boosted WE system (FOR-WE) is constructed by using PdPt BMLs as the cathode and the anode. The FOR-WE system only requires an operational voltage of 0.31 V to achieve H2 production, which is 1.48 V lower than that (ca. 1.79 V) with the use of the traditional WE system.
1 INTRODUCTION
Hydrogen (H2), a sustainable and environmentally friendly energy carrier, is a highly promising alternative to fossil fuels. Among various H2 production methods, water electrolysis (WE) is one of the most promising techniques for high-purity H2 production.1-8 However, the energy conversion efficiency of traditional WE is highly restricted by the sluggish oxygen evolution reaction (OER) at the anode, which expends ca. 90% electricity during the WE technique. Recently, liquid hydrocarbon fuel molecule (i.e., alcohols, carboxylic acids, sugars, etc.) electrooxidation reaction-boosted WE has attracted increasing attention, which effectively promotes the energy conversion efficiency of WE by decreasing the operational voltage due to the low thermodynamic oxidation potentials of liquid hydrocarbon fuel molecules.9-13 Among these small-molecule electrooxidation reactions, the two electron-transferred formate oxidation reaction (FOR) is the simplest in an alkaline medium. Meanwhile, FOR has lower thermodynamic oxidation potential and faster reaction kinetics than the formic acid electrooxidation reaction in an acidic medium (φHCOO‒/CO32‒ = ‒0.93 V vs. φHCOOH/CO2 = ‒0.22 V).14 Like formic acid, formate/water can also serve as an efficient hydrogen carrier, which has the advantages of nonflammable property, nontoxicity, high stability, and convenient storage/transportation.15 As a result, FOR-boosted WE (FOR-WE) may be an environmentally friendly and energy-saving method for high-purity H2 production.
FOR-WE consists of the anodic FOR and the cathodic hydrogen evolution reaction (HER). At present, palladium (Pd)-based nanostructures are the most active FOR electrocatalysts, whereas platinum (Pt)-based nanostructures are the most active HER electrocatalysts in an alkaline medium. Although formate has a very low thermodynamic oxidation potential, the overpotential of FOR in highly active Pd nanostructures is still as high as 0.2–0.4 V.15, 16 For development of the FOR-WE system, the fabrication of robust anode electrocatalysts with small FOR overpotential is important. The electroactivity of noble-metal-based nanostructures can be effectively increased by modifying their geometric structures, such as by the formation of two-dimensional (2D) metallenes (MLs, a class of graphene-like metal nanosheets).17-29 Generally, 2D MLs have a high specific surface area and ample unsaturated surface atoms with high activity, which provide high electroactivity and stability in electrochemical reactions.17-29 For example, Prof. Jin30 developed 2D Pd metallenes (Pd MLs) with 1.6 nm thickness using an etching-assisted reduction route, which showed 3.7-fold enhancement in mass electroactivity relative to the Pd/C electrocatalyst. Composition engineering is another efficient strategy for improving the electroactivity of noble-metal nanostructures due to the ensemble effect, the electronic effect, the third-body effect, the bifunctional mechanism, and so forth.31-35 For example, Prof. Chen35 developed PdAgRh alloy trimetallenes using the coreduction method, which showed 4.74-fold enhancement in FOR mass electroactivity compared to the Pd/C catalyst because of a unique 2D structure, the oxophilic property of Rh atoms, and the formation a Pd/Ag–O interface.
Recently, bimetallic PdPt nanostructures have been widely applied in electrocatalysis, which generally show enhanced electrocatalytic performance for many important electrochemical reactions (such as glycerol oxidation reaction,36 oxygen reduction reaction,37 OER,38 methanol oxidation reaction,39 formic acid oxidation reaction,40, 41 ethanol oxidation reaction,39 HER,42-44 etc.) because of the synergistic effect. For example, Prof. Chen45 synthesized high-quality AgPd–Pt aerogels using a kinetically controlled growth method, which revealed about fivefold enhancement of FOR mass electroactivity compared to the Pt/C catalyst because of efficient electronic structure modulation. In this work, we successfully synthesized PdPt bimetallenes (BMLs) using the galvanic replacement reaction strategy. The introduction of Pt sharply boosts the FOR electroactivity of PdPt BMLs and simultaneously endows PdPt BMLs with excellent HER electroactivity in an alkaline medium. Subsequently, a two-electrode FOR-WE system was constructed using bifunctional PdPt BMLs as the anode and the cathode that required an operational voltage of only 0.31 V to achieve H2 production, which is lower than that (ca. 1.79 V) of the traditional WE system.
2 EXPERIMENTAL SECTION
2.1 Reagents and chemicals
Potassium chloropalladite (K2PdCl4), potassium platinochloride (K2PtCl4), potassium hydroxide (KOH), potassium formate (HCOOK), and ferric trichloride (FeCl3) were purchased from Aladdin Industrial Corporation. Commercial Pt nanocrystals (Pt cNCs), commercial IrO2 nanocrystals (IrO2 cNCs), and commercial Pd nanocrystals (Pd cNCs) were obtained from Johnson-Matthey Corporation. Deionized water was used from the Milli-Q Integral Ultrapure water machine in all our experiments.
2.2 Synthesis of Pd MLs
Pd MLs were synthesized according to a previously reported oxidation-etching method, with slight modifications.43 In a typical synthesis, 15 mL of FeCl3 (61.5 mM) and 19.72 mL of K2PdCl4 (38.2 mM) were added in 200 mL of water. Pd MLs were generated by bubbling CO gas (0.3 L min−1) in a homogeneous FeCl3–K2PdCl4 mixture for 3 min. After the reaction, Pd MLs were collected by centrifugation and washed with a mixture of ethanol and water.
2.3 Synthesis of PdPt BMLs
In a typical synthesis, 1.0 mL of 0.15 M of K2PtCl4 solution was added to 8.0 mL of a Pd ML suspension (2 mg mL‒1) under stirring. After heating for 3 h at 60°C, PdPt BMLs were obtained by centrifugation and washed with a mixture of ethanol and water.
2.4 Physical/electrochemical characterizations and theoretical calculation
The experimental details of electrochemical measurement, physical characterization, and DFT calculation are provided in File S1.
3 RESULTS AND DISCUSSION
3.1 Physical characterization of PdPt BMLs
PdPt BMLs were obtained using a facile two-step synthesis procedure. First, Pd MLs were obtained using the previously reported oxidation-etching method, with slight modifications.43 The successful preparation of Pd MLs was corroborated by various physical characterizations, including scanning electron microscope (SEM), transmission electron microscope (TEM), powder X-ray diffractometer (PXRD), and so on (Figure S1). Subsequently, a K2PtCl4 solution was added to the suspension of Pd MLs. Due to the discrepancy in their standard electrode potential (φK2PtCl4/Pt = +0.755 V vs. φK2PdCl4/Pd = +0.591 V),46 the K2PtCl4 reaction precursor is reduced by Pd atoms at Pd MLs and newly generated Pt atoms bond to the surface of Pd MLs. Energy-dispersive X-ray spectroscopy (EDX) characterization shows that the atomic ratio of Pd/Pt in PdPt BMLs is 91.91:8.09 (Figure 1A), which is close to the result obtained by inductively coupled plasma mass spectrometer measurements (the atomic ratio of Pd/Pt is 92.61:7.39). The PXRD diffraction peaks of PdPt BMLs are the same as those of Pd MLs, in agreement with the standard diffraction peaks of Pd crystal (JCPDS-46-1043; Figure 1B). Also, no diffraction peaks of Pt crystals (JCPDS-04-0802) are observed in the PXRD pattern of PdPt BMLs due to the low Pt content. X-ray photoelectron spectrum (XPS) measurements were carried out to analyze the surface composition of PdPt BMLs. The XPS survey spectrum of PdPt BMLs reveals that the atomic ratio of Pd/Pt is 86.4:13.6 (Figure S2), which is lower than that obtained using the EDX technique. Mainly, the XPS technique has a shallower sampling depth than the EDX technique. The obvious discrepancy between EDX and XPS data hints at the formation of Pd core PdPt shell nanostructures.
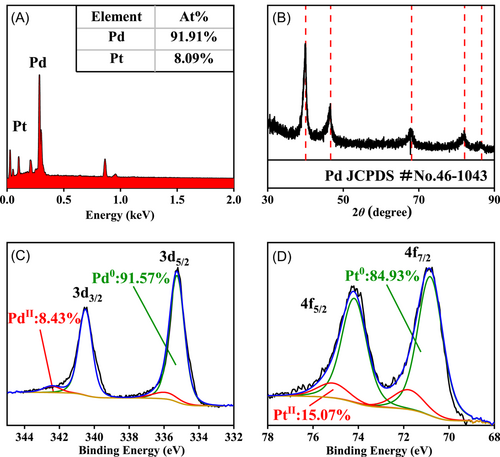
The deconvoluted Pd 3d XPS (Figure 1C) and Pt 4f XPS (Figure 1D) data indicate that both Pd and Pt atoms exist mainly in a metallic state, in which the contents of Pd0 and Pt0 species are estimated to be 83.72% and 84.93%, respectively. PdPt BMLs are nanoscale and are easily oxidized by oxygen when exposed to air, yielding metal oxides. Relative to the standard binding energy (BE) value of metallic Pd (3d5/2 = 335.10 eV), the BE value of Pd0 species (3d5/2 = 335.24 eV) in PdPt BMLs positively shifts ca. 0.14 eV. In contrast, the BE value of Pt0 species (4f7/2 = 71.12 eV) in PdPt BMLs negatively shifts ca. 0.18 eV compared to the standard BE value of metallic Pt (4f7/2 = 71.30 eV).47 The obvious shift in BE indicates electron migration from Pd atoms to Pt atoms due to differences in their electronegativity (Pd: 2.20 vs. Pt: 2.28).48 Consequently, the charge transfer between Pd atoms and Pt atoms may affect the electroactivity of PdPt BMLs because of the electronic effect.
The morphological features of PdPt BMLs were investigated by SEM (Figure 2A) and TEM (Figure 2B). SEM and TEM images reveal that PdPt BMLs show a nonlayered 2D morphology, which consist of 2D nanosheets of ca. 150 nm. This nonlayered morphology of PdPt BMLs can effectively overcome the stacking tendency of 2D nanosheets, endowing PdPt BMLs with a high accessible surface area. So far, SEM and TEM characterizations have shown that the morphology of PdPt BMLs is very close to that of Pd MLs. The present experimental results suggest that the galvanic replacement reaction between K2PtCl4 and Pd MLs is mild, which only introduces Pt atoms on the surface of Pd MLs, but does not alter their initial 2D morphology.
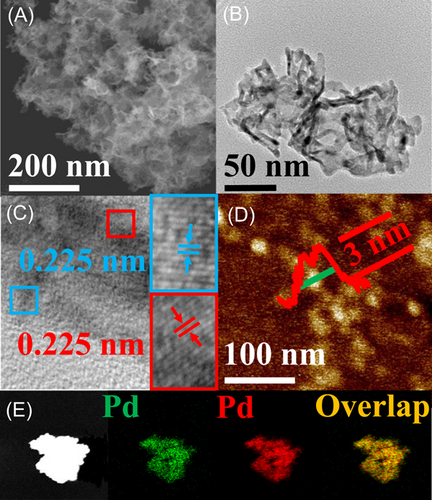
The fine structural feature of PdPt BMLs was characterized by a high-resolution TEM (HR-TEM) measurement, in which the image of a representative edge on nanosheet subunits was found. The HR-TEM image reveals clear and continuous fringes in the same direction (Figure 2C), which in turn suggests that Pt atoms have entered the lattice of Pd to generate a PdPt alloy at the surface of Pd MLs. The alloying phenomenon is very common during the galvanic replacement reaction, in which the thermodynamic driving force is responsible for the formation of an alloy due to the minimization of surface energy.43 Additionally, the HR-TEM image reveals that the lattice spacing is 0.225 nm, close to the lattice spacing of the Pd (111) crystallographic plane, suggesting that Pt atoms only exist at the surface of Pd MLs rather than diffuse into the bulk of Pd MLs (Figure 2C). Atom force microscope (AFM) measurement reveals that the thickness of PdPt BMLs is about 3 nm (Figure 2D). Furthermore, EDX maps were constructed to investigate the element distribution of PdPt BMLs. The patterns of EDX maps reveal that the patterns of Pt and Pd elements on PdPt BMLs are very uniform and similar, indicating their uniform distribution (Figure 2E). The EDX line-scan pattern also suggests the alloy structure of PdPt BMLs (Figure S3). Thus, all physical characterizations indicate that PdPt BMLs have an enriched PdPt alloy surface. Generally, Pt nanostructures are highly active electrocatalysts for the methanol electrooxidation reaction. However, PdPt BMLs are completely inactive for the methanol electrooxidation reaction (Figure S4), which suggests that no Pt nanoparticles exist on the surface of PdPt BMLs.
3.2 FOR electroactivity of PdPt BMLs
The electrochemical properties of Pd MLs, PdPt BMLs, and commercial Pt nanocrystals (Pt cNCs) were investigated by cyclic voltammetry (CV) tests (Figure 3A). In this work, all potentials were converted into the reversible hydrogen electrode (RHE). Compared to Pd MLs, PdPt BMLs show a smaller reduction peak of Pd oxide, which may be ascribed to the partial replacement of Pd atoms by Pt atoms at the surface of PdPt BMLs. Meanwhile, PdPt BMLs have a much bigger H absorption/desorption peak area than Pd MLs, which indicates that the formation of the PdPt alloy facilitates the H absorption in Pd materials.
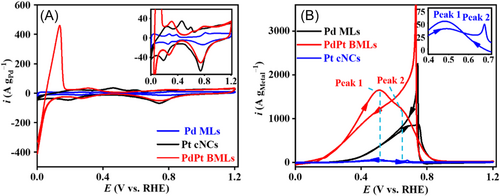
The electrocatalytic performances of Pd MLs, PdPt BMLs, and Pt cNCs were investigated by CV measurements in 0.5 M HCOOK + 1.0 M KOH electrolyte (Figure 3B). The CV curve of PdPt BMLs shows two distinct oxidation peaks (the main Peak 1 at 0.50 V and the shoulder Peak 2 at 0.68 V), which corresponds to the direct pathway and indirect pathway of FOR, respectively.49 The similarity in CV curves of PdPt BMLs and Pt cNCs indicates that the high FOR activity of PdPt BMLs mainly originates from the Pt active sites. Although the FOR peak potential at Pt cNCs is more negative than that at Pd MLs, the FOR peak current at Pt cNCs is 21.31 times lower than that at Pd MLs at 0.50 V. This fact indicates that Pt atoms in pure Pt mental have very low FOR electroactivity in an alkaline medium relative to Pd metal. Interestingly enough, Pt atoms in the PdPt alloy show high FOR electroactivity. The FOR peak current at PdPt BMLs is higher than that at Pd MLs and the FOR peak potential at PdPt BMLs negatively shifts ca. 224 mV relative to Pd MLs, revealing a significantly enhanced FOR electroactivity. At 0.50 V potential, FOR current at PdPt is 1649.4 A g−1, which is about 4.53 and 41.63 times higher than that at Pd MLs (363.8 A g−1) and Pt cNCs (39.57 A g−1), respectively. Additionally, PdPt BMLs also have much better FOR electroactivity than Pd cNCs (Figure S5) and various reported Pt- and Pd-based electrocatalysts (Table 1), further confirming the high mass electroactivity of PdPt BMLs for FOR.
| Catalyst | Electrolyte | Onset potential (V vs. RHE) | Mass activity at 0.50 V (A g−1) | Specific activity at 0.50 V (A m−2) | Ref. |
|---|---|---|---|---|---|
| PdPt BMLs | 1.0 M KOH | 0.085 | 1649.4 | 75.34 | This work |
| 0.5 M HCOOK | |||||
| Stream treatment Pd/CeO2 | 1.0 M NaOH | ca. 0.38 | ca. 250 | ca. 5.1 | [50] |
| 1.0 M HCOOK | |||||
| AgPd–AgF heterointerfaces | 1.0 M KOH | ca. 0.23 | ca. 150 | ca. 10.3 | [51] |
| 1.0 M HCOOK | |||||
| Ag30Pd69Co1 nanosheets | 1.0 M KOH | ca. 0.22 | ca. 500 | ca. 6.7 | [52] |
| 1.0 M HCOOK | |||||
| Pd hydride nanodendrites | 1.0 M KOH | ca. 0.22 | ca. 200 | ca. 28.8 | [15] |
| 0.5 M HCOOK | |||||
| AgPdF nanoalloys | 1.0 M KOH | ca. 0.40 | ca. 300 | ca. 10.3 | [53] |
| 1.0 M HCOOK | |||||
| Pd85Sn15/C | 1.0 M KOH | ca. 0.15 | ca. 450 | ca. 43.7 | [54] |
| 1.0 M HCOOK |
- Abbreviations: BML, bimetallene; FOR, formate oxidation reaction; RHE, reversible hydrogen electrode.
To understand the high mass electroactivity of PdPt BMLs for FOR, CO-stripping tests were performed (Figure 4A). According to the oxidation charge of preadsorbed CO, the electrochemically active surface area (EASA) values of Pd MLs, PdPt BMLs, and Pt cNCs are calculated to be 19.48, 21.89, and 11.15 m2 g–1, respectively. Obviously, the area effect is not the main factor responsible for the high mass activity of PdPt BMLs. Meanwhile, the onset potential and the peak potential of preadsorbed CO at PdPt BMLs are more negative than those at Pd MLs but more positive than those at Pt cNCs, indicating that the introduction of Pt can facilitate the CO oxidation at the Pd surface in an alkaline medium.
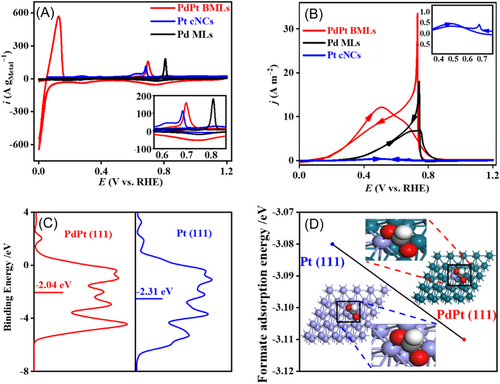
After normalizing FOR current to EASA, the peak current densities of FOR at PdPt BMLs, Pd MLs, and Pt cNCs are 75.34, 74.28, and 4.93 A m‒2, respectively (Figure 4B). At 0.50 V potential, FOR current density at PdPt BMLs (75.34 A m‒2) is still about 2.93 and 15.91 times higher than that at Pd MLs (25.63 A m‒2) and Pt cNCs (4.74 A m‒2), respectively. Thus, EASA-normalized CV curves indicate that PdPt BMLs have higher intrinsic electroactivity than Pd MLs and Pt cNCs, which contributes to the high mass electroactivity of PdPt BMLs for FOR.
Density functional theory (DFT) calculations were performed to examine the high electroactivity of PdPt BMLs. According to the HR-TEM result, PdPt (111) and Pt (111) planes were simulated. Then, the detailed partial density of states of the Pt d-state on PdPt (111) and Pt (111) planes are calculated, which indicates that there is a higher density of states closer to the Fermi level and a narrower Pt d state on the PdPt (111) plane with respect to that on the Pt (111) plane (Figure 4C). Furthermore, the d-band center energy of Pt on the PdPt (111) plane (−2.04 eV) is also revealed, which is significantly higher than that (−2.31 eV) on the Pt (111) plane. These results indicate a modified Pt electronic structure on the PdPt (111) plane. Herein, the strong interaction induced by the Pd−Pt bond can strengthen the bonding of Pt to adsorbed species due to the upshifted d-band center of Pt. Theoretically, the atomically dispersed Pt atoms on the surface of PdPt BMLs will undergo electronic structure modification due to the surrounding Pd atoms, which may show special adsorption performance. As expected, the Gibbs free energy of formate adsorption ΔG(HCOO) on Pt is −3.11 eV for the PdPt (111) plane and −3.08 eV for the Pt (111) plane (Figure 4D). Enhanced ΔG(HCOO*) means that HCOO* is more easily activated and further oxidized on Pt active sites of the PdPt (111) plane. Specifically, the adsorption of HCOO* is strengthened, whereas the adsorption of H and COads is weakened (Figure 4D), resulting in improved FOR kinetics and high intrinsic activity of PdPt BMLs. Therefore, the interaction between Pd and Pt optimizes the electronic structure of Pt in PdPt BMLs, which can reduce the reaction energy barrier of FOR, resulting in a remarkably boosted FOR electroactivity. Generally, the chemical composition strongly affects the electroactivity. Thus, PdPt BMLs with different atomic ratios of Pd/Pt were synthesized using the same galvanic replacement reaction procedures (Figure S6). Further CV measurements reveal that PdPt BMLs have obvious component-dependent electroactivity for FOR (Figure S7). Among them, the present PdPt BMLs with 8.09% Pt have the best FOR electroactivity. This fact indicates that the synergistic effect between Pt atoms and Pd atoms plays a very important role in the high electroactivity of PdPt BMLs again.
The FOR durability of Pd MLs and PdPt BMLs was estimated by chronoamperometry tests (Figure S8). FOR current at Pd MLs quickly decreases to 0 over 10,000 s due to low electroactivity. Compared to Pd MLs, PdPt BMLs show a slower FOR current attenuation. At 8000 s, the FOR current density at PdPt BMLs is 45.83 A g−1, which is 11.66 times higher than that at Pd MLs (3.93 A g−1). Obviously, the improved antipoisoning capability of PdPt BMLs for CO intermediate oxidation contributes to the high stability of PdPt BMLs for FOR. After the long-term chronoamperometry test, the morphology of PdPt BMLs was investigated by TEM (Figure S9). Experimental data show that the unique 2D structure of PdPt BMLs can still be preserved, indicating the chemical stability of PdPt BMLs.
3.3 HER electroactivity of PdPt BMLs
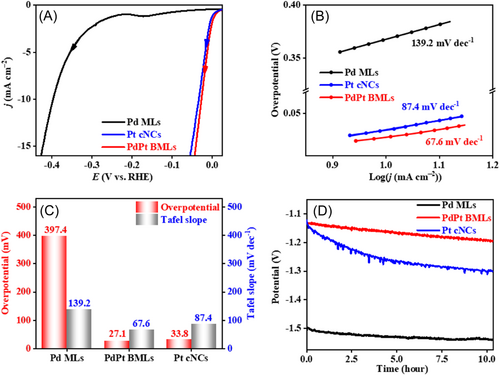
HER electroactivities of Pd MLs, PdPt BMLs, and Pt cNCs were assessed by linear sweep voltammetry (LSV), in which 95% iR-correct was applied and current density was normalized to the electrode area of the working electrode. The HER overpotentials at Pd MLs, PdPt BMLs, and Pt cNCs are 394.9, 27.1, and 33.8 mV at 10 mA cm‒2, respectively (Figure 5A). Tafel slopes of HER at Pd MLs, PdPt BMLs, and Pt cNCs are determined to be 139.2, 67.6, and 87.4 mV dec−1, respectively (Figure 5B). As observed, PdPt BMLs show much smaller HER overpotential and Tafel slope values compared to Pd MLs due to the introduction of the Pt element (Figure 5C), which is sufficiently close to those at Pt cNCs. Obviously, Pt atoms at PdPt BMLs are the active sites for HER. Meanwhile, the HER electroactivity of PdPt BMLs is also comparable to those of various reported advanced Pt-based and Pd-based electrocatalysts (Table 2). According to DFT calculations, the upward shift of the d-band center of PdPt can enhance the adsorption capacity of Had, which effectively improves the intrinsic activity of HER (Figure 4C). Meanwhile, the unique 2D structure of PdPt BMLs can provide enough reaction sites, which is conducive to the adsorption of Had and the escape of H2. Due to the enhanced adsorption capacity of Had and the unique 2D structure, PdPt BMLs show much smaller HER overpotential and Tafel slope values compared to Pd MLs and Pt cNCs. The stability of HER at Pd MLs, PdPt BMLs, and Pt cNCs was investigated by chronopotentiometry tests. Compared with Pt cNCs, PdPt BMLs show a smaller potential increase within 10 h (Figure 5D). Since such a low applied potential cannot result in oxidation etching of PdPt BMLs, the excellent HER durability of PdPt BMLs can be ascribed to the 3D self-supporting architecture of PdPt BMLs.
| Catalyst | Electrolyte | η10 (mV) | Tafel slope (mV dec−1) | Ref. |
|---|---|---|---|---|
| PdPt MLs | 1.0 M KOH | 27.1 | 67.6 | This work |
| Pt-decorated NiO microflowers | 1.0 M KOH | 66 | 82 | [55] |
| Pd-doped hollow Ru–Te nanorods | 1.0 M KOH | 37 | 61.6 | [56] |
| Pd–CeO2−x-N-doped carbon nanosheets | 1.0 M KOH | 115 | 58 | [57] |
| N-doped Pd/amorphous–cobalt (II) interface | 1.0 M KOH | 58 | 71.3 | [53] |
| NiPd alloy | 1.0 M KOH | 38 | 168 | [58] |
| Pd1–CoSe2 nanobelts | 1.0 M KOH | 80 | 92 | [59] |
| Pd/NiFeOx nanosheets | 1.0 M KOH | 42 | 78.0 | [60] |
| PdSe2 nanosheets | 1.0 M NaOH | 138 | 100 | [61] |
| Pt/NiO/Nion carbon nanotubes | 0.1 M KOH | 350 | 72 | [62] |
| Pt-doped Fe alloy | 1.0 M KOH | 217 | 90.1 | [62] |
| N-doped PdCoNi carbon-based nanosheets | 1.0 M KOH | 147 | 67 | [63] |
- Abbreviations: HER, hydrogen evolution reaction; ML, metallene.
3.4 FOR-WE system
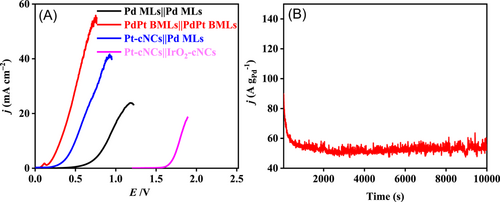
To achieve H2 generation from an aqueous formate electrolyte, a two-electrode PdPt BMLs||PdPt BMLs electrolyzer was constructed. LSV curves show that PdPt BMLs||PdPt BMLs formate electrolyzer requires a voltage of only 0.31 V to achieve 10 mA cm−2, which is 1.48 V lower than the voltage of 1.79 V in a Pt cNCs||IrO2 cNCs water electrolyzer (Figure 6A). Meanwhile, the electrolysis voltage in the PdPt BMLs||PdPt BMLs formate electrolyzer (0.31 V) is also smaller than those in the Pd MLs||Pd MLs formate electrolyzer (0.90 V) and the Pt cNCs||Pd MLs formate electrolyzer (0.50 V) at 10 mA cm−2, which can be ascribed to the excellent electroactivity of PdPt BMLs for both FOR and HER (Figure 6A). Such a small electrolysis voltage of the PdPt BMLs||PdPt BMLs electrolysis system is also lower than those of various reported small-molecule oxidation-boosted WE systems (Table 3) as well as other formate electrolysis systems for electrochemical H2 production (Table 4).
| Substrate molecule | Anode material | Cathode material | Electrolysis voltage at 10 mA cm‒2 | Ref. |
|---|---|---|---|---|
| Formate | PdPt BMLs | PdPt BMLs | 0.31 V | This work |
| Ethanol | Ni/Y0.16Zr0.84O1.92 cermet | Ni/Y0.16Zr0.84O1.92 cermet | 1.30 V | [64] |
| 5-Hydroxymethylfurfural | CoNiP nanosheet | CoNiP nanosheet | 1.30 V | [65] |
| Urea | Rh/NiV-layered double hydroxide | Rh/NiV-layered double hydroxide | 1.33 V | [66] |
| Glucose | RuCoMn nanoparticle | RuCoMn nanoparticle | 1.63 V | [67] |
| Methanol | Mesoporous PtRh nanosheets | Mesoporous PtRh nanosheets | 0.51 V | [68] |
| Formaldehyde | RhPb nanoflowers | RhPb nanoflowers | 0.57 V | [69] |
| Formic acid | 1, 10-Phenanthroline monohydrate-functionalized Pt nanodendrites | Polyethylenimine-functionalized Pt- nanodendrites | 0.45 V | [70] |
| Isopropanol | Rh nanoroses | Graphite | 0.33 V | [71] |
| Benzylamine | Ultra-thin Ni(OH)2 nanomeshes | Ultra-thin Ni(OH)2 nanomeshes | 1.41 V | [72] |
| Benzyl alcohol | Hierarchical Mo–Ni nanoparticle | Hierarchical Mo–Ni nanoparticle | 1.36 V | [73] |
- Abbreviation: BML, bimetallene.
| Anode material | Cathode material | Substrate molecule | Cell voltage (V) | Ref. |
|---|---|---|---|---|
| PdPt BMLs | PdPt BMLs | Formate | 0.31 | This work |
| PdNi bimetallene | PdNi bimetallene | Formate | 0.51 | [69] |
| PdMn bimetallene | PdMn bimetallene | Formate | 0.38 | [74] |
| S–NiCo-layered double hydroxide | S–NiCo-layered double hydroxide | Formate | 1.39 | [75] |
| Cu0.33Co Co-layered double hydroxide | Cu0.33Co Co-layered double hydroxide | Formate | 1.34 | [76] |
| Boron-incorporated Pd | Commercial Pt/C | Formate | 0.79 | [77] |
- Abbreviation: BML, bimetallene.
The long-term stability of a PdPt BMLs||PdPt BMLs formate electrolyzer was studied using a chronoamperometry test (Figure 6B). The PdPt BMLs||PdPt BMLs formate electrolyzer shows a slow current attenuation at a voltage of 0.31 V. After 10,000 s, the PdPt BMLs||PdPt BMLs formate electrolyzer can still achieve a current density of 54.3 A g−1 for continuous H2 production, indicating its good durability in the FOR-WE system. Obviously, the excellent electroactivity and stability of PdPt BMLs for both FOR and HER contribute toward the outstanding durability of the PdPt BMLs||PdPt BMLs formate electrolyzer.
4 CONCLUSION
PdPt BMLs were successfully synthesized using a galvanic replacement reaction strategy. Electrochemical data indicated that the FOR electroactivity of PdPt BMLs directly correlated with the atomic ratio of Pd/Pt. PdPt BMLs with an optimized composition revealed enhanced electroactivity for FOR compared to Pd MLs due to the synergistic effect of the PdPt alloy. Specifically, the peak potential of FOR at PdPt BMLs revealed a negative shift of 224 mV relative to Pd MLs. Meanwhile, PdPt BMLs also showed enhanced durability for FOR compared to Pd MLs because of the improved CO tolerance. Additionally, the introduction of the Pt element also greatly enhanced the electroactivity of PdPt BMLs for HER, which even exceeded the electroactivity of Pt cNCs for HER. Because of the excellent electroactivity and stability of PdPt BMLs for both FOR and HER, a PdPt BMLs||PdPt BMLs two-electrode electrolysis system was constructed for electrochemical H2 generation, which needs an electrolysis voltage of only 0.31 V for H2 production at 10 mA cm‒2. Indeed, the electrolysis voltage of the PdPt BMLs||PdPt BMLs formate electrolyzer was 1.48 V lower than that of the Pt cNCs||IrO2 cNCs water electrolyzer, revealing a highly energy-saving electrochemical strategy for H2 production.
ACKNOWLEDGMENTS
This research was sponsored by the National Natural Science Foundation of China (22272103), the Shenzhen Stable Supporting Program (20220716001753001 and SZWD2021015), the University Engineering Research Center of Crystal Growth and Applications of Guangdong Province (2020GCZX005), the Science and Technology Innovation Team of Shaanxi Province (2023-CX-TD-27 and 2022TD-35), the Fundamental Research Funds for the Central Universities (GK202202001), and the 111 Project (B14041).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.




