Association between plasma growth differentiation factor 15 levels and pre-eclampsia in China
Shuhong Xu and Yicheng Lu contributed equally to this study.
Abstract
Background
Growth differentiation factor-15 (GDF-15) is a stress response protein and is related to cardiovascular diseases (CVD). This study aimed to investigate the association between GDF-15 and pre-eclampsia (PE).
Method
The study involved 299 pregnant women, out of which 236 had normal pregnancies, while 63 participants had PE. Maternal serum levels of GDF-15 were measured by using enzyme-linked immunosorbent assay kits and then translated into multiple of median (MOM) to avoid the influence of gestational week at blood sampling. Logistic models were performed to estimate the association between GDF-15 MOM and PE, presenting as odd ratios (ORs) and 95% confidence intervals (CIs).
Results
MOM of GDF-15 in PE participants was higher compared with controls (1.588 vs. 1.000, p < 0.001). In the logistic model, pregnant women with higher MOM of GDF-15 (>1) had a 4.74-fold (95% CI = 2.23–10.08, p < 0.001) increased risk of PE, adjusted by age, preconceptional body mass index, gravidity, and parity.
Conclusions
These results demonstrated that higher levels of serum GDF-15 were associated with PE. GDF-15 may serve as a biomarker for diagnosing PE.
Key points
-
Growth differentiation factor-15 (GDF-15) is associated with increased risk of pre-eclampsia.
-
GDF-15 may be a potential biomarker of pre-eclampsia.
1 INTRODUCTION
Pre-eclampsia (PE) is a pregnancy-specific syndrome that usually occurs after 20 weeks of gestation. It is characterized by hypertension and proteinuria, which may also be related with other symptoms such as edema, vision loss, and headache.1 PE affects approximately 2%–8% of pregnancies globally1 and 4.5%2 in China, contributing to high maternal and perinatal morbidity and mortality.3 If left untreated, PE can progress to severe PE and hemolysis, elevated liver enzymes, and low platelets syndrome. PE can bring multiple maternal complications including hypertensive retinopathy, pulmonary edema, placental abruption, or even stroke, as well as fetal complications such as fetal growth restriction, premature delivery, oligoamnios, and small-for-gestational-age infants.4, 5 Moreover, there is strong evidence that women who develop PE are at increased risk of long-term cardiovascular complications in later life.6, 7 Furthermore, their offspring are more likely to experience neurodevelopmental problems due to preterm birth during childhood.7, 8 Therefore, it is of great clinical and health significance to explore the pathogenesis of PE and thus to early diagnosis and intervention of PE.
The “two-stage model” is widely accepted as the primary theory for the mechanism of PE.9 The primary cause of PE is defective trophoblast invasion, which can lead to abnormal placenta development and therefore impaired placental perfusion.9 Poorly perfused placentas progressively produce antiangiogenic factors such as sFlt-1 and sEng, which leads to the second stage of PE—general endothelial dysfunction and vascular inflammation that lead to systemic organ damages.9, 10 Recently, the Fetal Medicine Foundation (FMF) proposed a Bayes theorem-based model to predict preterm PE at 11–13 weeks' gestation by using a combination of maternal characteristics, medical history, mean arterial pressure, uterine artery pulsatility index, and serum placental growth factor.11 Subsequently, the identified high-risk women were recommended to take low-dose aspirin prophylactic therapy due to its antiplatelet and anti-inflammatory effects.12 However, the detection rate of FMF model was limited, only about 75% of preterm PE and 43% of term PE were detected at a 10.0% false positive rate.13 Moreover, a large-scale randomized controlled trial in China demonstrated even under the preventive treatment of low-dose aspirin, the rate of PE was still up to 16.8%.14 Therefore, there is still a need to improve early screening and intervention programs for PE.
Growth differentiation factor 15 (GDF-15), also called macrophage inhibitory cytokine-1 (MIC-1), belongs to the transforming growth factor-beta family cytokines. It is a stress response protein that is significantly induced in various stressful conditions, such as inflammation and ischemia-reperfusion injury.15 The protective role of GDF-15 against myocardial injury and pressure cardiac overload by preventing cell death, cardiac dilation, and hypertrophy has been observed in animal models.16, 17 It has also been reported that GDF-15 can protect endothelial cells by releasing NO when inducted by oxidized low-density lipoprotein (ox-LDL), which is involved in the inflammatory process of atherosclerosis.18 In addition, clinical studies have found that this biomarker is positively correlated with an increased risk of cardiovascular events and is considered an independent predictor of mortality in patients with coronary artery disease and heart failure.19-21 Moreover, increased circulating levels of GDF-15 have also been observed in women with gestational diabetes mellitus,22-24 possibly due to inflammation and immune activation.25
Although the causes of PE are not yet fully understood, it is known that the pathogenesis of PE involves endothelial dysfunction and systematic inflammatory response,4 in which GDF-15 is involved. A meta-analysis26 including 498 women with PE and 2349 women with normal pregnancy showed that compared to controls of women with normal pregnancy, women with PE had significantly higher circulating GDF-15. However, relevant evidence in China is still scarce.
Therefore, the objective of this study was to compare serum levels of GDF-15 between women with PE and healthy normal controls among Chinese.
2 METHOD
2.1 Sample collection
A total of 236 healthy pregnancies and 63 PE cases who had regular prenatal care in the first People's Hospital in Taicang between June 2021 and July 2022 were included in this study. Inclusion criteria for subjects were (1) age ≥20 years, (2) willingness to participate in the study and sign the informed consent, and (3) singleton pregnancy. Exclusion criteria for subjects were (1) history of diabetes; (2) gestational diabetes; (3) patients with autoimmune disease, thyroid disease, heart disease, and liver or kidney disease; (4) cancer patients; and (5) patients with blood diseases.
PE was defined as the new onset of hypertension (systolic and/or diastolic blood pressure of ≥140 and/or 90 mmHg) after the 20th week of pregnancy concomitant proteinuria (≥300 mg in a 24-h urine or 1[+] protein by dipstick test on two random urine samples).1 PE was grouped into early-onset (<34 weeks, n = 13) and late-onset (≥34 weeks, n = 50) PE based on the gestational week of diagnosis. The basic information of the participants was collected by the questionnaires under the guidance of professional medical staff, including maternal age, height, weight before pregnancy, history of hypertension, family history of hypertension, parity, and gravidity.
All participants provided written informed consent, and the study was approved by the ethics committee of the (redacted) University.
2.2 GDF-15 enzyme-linked immunosorbent assay (ELISA)
Blood samples were taken at the time of PE diagnosis for cases and between 24 and 38 gestational weeks for controls. Serum GDF-15 levels were measured using a quantitative sandwich enzyme-linked immunosorbent assay kit (Quantikine ELISA kit for human GDF-15; Catalog No. DGD150; R&D Systems). The coefficient of variation was <3% for intra-assay precision and <6% for inter-assay precision. Data less than the minimum of machine measurement is replaced by half of the measured lowest concentration.
2.3 Statistical analysis
Continuous variables with normal distribution were represented as mean ± standard deviation (SD), while numerical variables with skewered distribution were represented by median (M) and 25th–75th percentile. For categorical variables, n (%) was described. Two samples t-test, Mann–Whitney U test, and chi-square test were used to compare the difference of variables between the PE and control groups, as appropriate. To reduce the influence of gestational week at blood sampling on levels of GDF-15, we used multiple of median (MOM) to represent the relative levels of GDF-15. MOM is defined as the level of GDF-15 divided by the median level of GDF-15 in the control group at the same gestational week. Due to the skewed distribution, GDF-15 concentrations were divided into two groups by MOM of 1, which is the median value of the controls. Next, to identify the association between GDF-15 and PE, univariable and multivariable logistic regression models were performed. Adjusted model 1 was controlled for age and preconceptional BMI. Adjusted model 2 was controlled for age, preconceptional BMI, gravidity, and parity. Two-tailed p < 0.05 was considered statistically significant. All statistical analyses were conducted with SAS version 9.4 (SAS Institute).
3 RESULTS
3.1 Demographic information of the study population
The clinical characteristics of PE and control groups are summarized in Table 1. There was no significant difference in age, height, parity, gravidity, and family history of hypertension between the two groups. However, women with PE had significantly higher weight and preconceptional BMI than controls (p < 0.001). Meanwhile, women with PE were more likely to have a cesarean section than controls (p < 0.001). About 11.3% of women in the PE group experienced chronic hypertension before pregnancy. As for fetal information, offspring of mothers with PE had significantly lower birthweight than normal pregnant women (p < 0.001) and were more likely to be low-birthweight infant (p < 0.001).
| Variants | Overall (n = 299) | Control (n = 236) | Pre-eclampsia (n = 63) | p-Value |
|---|---|---|---|---|
| Mother | ||||
| Age (years) | 29.829 ± 4.197 | 29.720 ± 3.845 | 30.238 ± 5.330 | 0.472 |
| Height (cm) | 160.472 ± 5.726 | 160.360 ± 5.739 | 160.889 ± 5.696 | 0.516 |
| Weight before pregnancy (kg) | 56.00 (51.00–62.00) | 55.00 (51.00–60.25) | 62.00 (53.50–74.75) | <0.001 |
| Preconceptional BMI (kg/m2) | 21.830 (19.895–24.220) | 21.485 (19.570–23.730) | 23.620 (20.835–27.620) | <0.001 |
| Delivery mode | <0.001 | |||
| Spontaneous vaginal delivery | 153 (51.1) | 139 (58.9) | 14 (21.9) | |
| Cesarean section | 146 (48.8) | 97 (41.1) | 49 (78.1) | |
| Serum GDF-15 (pg/mL) | 43,344 (28,432–65,968) | 39,822 (27,414–56,744) | 94,640 (55,440–140,740) | <0.001 |
| MOM of GDF-15 | 1.070 (0.740–1.540) | 1.000 (0.679–1.327) | 1.588 (1.104–2.667) | <0.001 |
| ≤1 | 126 | 115 (48.7) | 11 (17.5) | <0.001 |
| >1 | 173 | 121 (51.3) | 52 (82.5) | |
| Family history of hypertension | ||||
| Yes | 251 (83.9) | 198 (83.9) | 53 (84.1) | 0.998 |
| No | 48 (16.1) | 38 (16.1) | 10 (15.9) | |
| History of hypertension | ||||
| Yes | 7 (2.3) | 0 (0) | 7 (11.3) | <0.001 |
| No | 292 (97.3) | 236 (100.0) | 56 (88.7) | |
| Gestational week at blood sampling (weeks) | 30 (28–34) | 28 (28–34) | 36 (34–37) | <0.001 |
| Gravidity | 0.186 | |||
| Nulligravid | 90 (30.1) | 73 (30.9) | 17 (27.0) | |
| Gravid | 209 (69.9) | 163 (69.1) | 46 (73.0) | |
| Parity | 0.544 | |||
| Nulliparous | 144 (48.2) | 109 (46.2) | 35 (55.6) | |
| Parous | 155 (51.8) | 127 (53.8) | 28 (44.4) | |
| Infant | ||||
| Birthweight (g) | 3185.74 ± 567.73 | 3321.12 ± 448.26 | 2661.97 ± 672.37 | <0.001 |
| Low birthweight (<2500 g) | 30 (10.0) | 9 (3.8) | 21 (38.1) | <0.001 |
| Normal birthweight (2500–4000 g) | 250 (83.6) | 211 (89.4) | 39 (57.1) | |
| Macrosomia (>4000 g) | 19 (6.3) | 16 (6.8) | 3 (4.8) |
- Note: Data are presented as n (%), mean (SE), or median (25th–75th percentile).
- Abbreviations: BMI, body max index; GDF-15, growth differentiation factor 15; MOM, multiple of median.
The median of GDF-15 levels was significantly different between controls and PE groups (39,822 pg/mL vs. 94,640 pg/mL, p < 0.001). Meanwhile, MOM of GDF-15 was significantly different between controls and PE groups (median: 1.000 vs. 1.588, p < 0.001). There was no significant difference between early-onset PE and late-onset PE (median: 1.221 vs. 1.774, p = 0.292). Box diagram in Figure 1 demonstrated the distribution of MOM of GDF-15 from early-onset PE, late-onset PE, PE, and control.
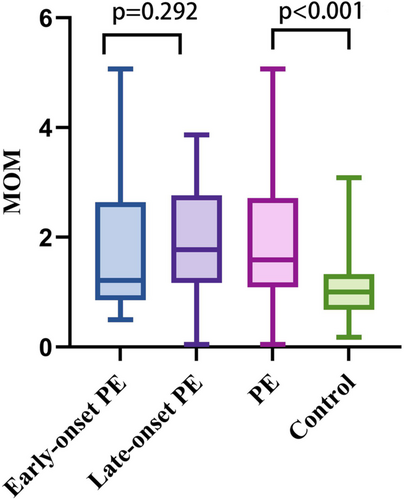
3.2 Association between GDF-15 and PE
As shown in Table 2, MOM of GDF-15 was positively associated with the risk of PE, yielding an odds ratio of 4.49 (95% confidence interval [CI] = 2.23–9.03) in the crude model. Results were stable in different multivariable models. In adjusted model 1, after controlling for age and preconceptional BMI, the association remained significant (odd ratio [OR] = 5.03, 95% CI = 2.39–10.58, p < 0.001). In adjusted model 2, the risk of PE in pregnant women with higher GDF-15 MOM (>1) increased by 4.74-fold (95% CI = 2.23–10.08, p < 0.001) after adjusted by age, preconceptional BMI, gravidity, parity.
| Modela | Odds ratio (95% CI) | p-Value |
|---|---|---|
| Crude model | 4.49 (2.23–9.03) | <0.001 |
| Adjusted model 1 | 5.03 (2.39–10.58) | <0.001 |
| Adjusted model 2 | 4.74 (2.23–10.08) | <0.001 |
- Note: Adjusted model 1: adjusted by age, preconceptional BMI. Adjusted model 2: adjusted by age, preconceptional BMI, gravidity, parity.
- Abbreviations: BMI, body max index; CI, confidence interval; GDF-15, growth differentiation factor 15; MOM, multiple of median.
- a Comparing MOM > 1 with MOM < 1.
4 DISCUSSION
The results of our study are consistent with previous studies including a meta-analysis,26-28 which showed that there is a positive link between GDF-15 and an increased risk of PE, indicating that GDF-15 may be used as a diagnostic biomarker for PE.
Although most studies have demonstrated that GDF-15 was related with increased risk of PE,26-28 Chen et al. found that serum GDF-15 was significantly reduced in the third trimester in women presenting with PE, especially in late-onset cases.29 Additionally, a study conducted in Australia showed a null association between circulating levels of GDF-15 and PE.30 A nested case–control study drawn from a prospective observational study found that there was no significant difference in levels of GDF-15 between preeclamptic women and controls at 19–24 or 30–34 or 35–37 gestational weeks.31 These differences may be due to variations in race, gestational age at sample collection, and sample size.
GDF-15 can exert anti-inflammatory effects by inhibiting macrophage activation,32 while the development of PE is often accompanied by inflammation and activation of the immune system.33 Based on this, it appears that GDF-15 may rise compensatively during the development of PE.
Similarly, studies have shown conflicting results on the association between GDF-15 and some other diseases. For instance, while epidemiological studies demonstrated that higher GDF-15 levels, as a response to tissue inflammation, are positively related with progression and poor prognosis of cardiovascular diseases,32, 34 recent animal studies suggested that GDF-15 could potentially provide cardioprotective benefits through the SMAD pathway.16, 17 Furthermore, GDF-15 has been identified to play a protective role in acute kidney injury and kidney fibrosis in mice,35, 36 but epidemiological studies have linked it to an increased risk of chronic kidney disease progression.37 These findings suggested that GDF-15 may be a biomarker rather than a causal factor for these diseases. Further investigations are needed to determine whether higher levels of GDF-15 occur as a compensatory response to tissue injury in PE or are simply bystanders.
It should be noted that our study has limitations regarding the lack of matching for gestational week at blood sampling between preeclamptic and control subjects. However, the application of MOM may minimize its impact. Besides, as with previous studies, not all potential confounders were adjusted, and the possibly unmeasured or unknown covariables could not be ruled out. Last, the observational design of our studies precludes establishing causality and the mechanism between GDF-15 and PE; further research is still needed.
In conclusion, higher levels of serum GDF-15 were found to be associated with PE, indicating its potential as a biomarker for diagnosing the condition. However, further research is needed to explore the mechanisms and diagnostic implications of these findings.
AUTHOR CONTRIBUTIONS
All authors have participated in the concept and design of this manuscript. Shuhong Xu, Jiani Qian, Songliang Liu, Shilan Yan, and Qiuping Ma participated in the collecting of blood samples. Shuhong Xu, Yicheng Lu, Jieyun Yin, Mengxin Yao, and Zhuoqiao Yang participated in the analysis and interpretation of data and drafting of the manuscript; the rest of the authors took part in the revising of the manuscript.
ACKNOWLEDGMENTS
This work was supported by the 2021 Taicang Basic Research Program (Medical and Health Applied Basic Research Project) with project number TC2021JCYL03 and the 2022 guiding project of the 28th batch of Suzhou Science and Technology Development Plan (Medical and Health Scientific and Technological Innovation) with project number SKYD2022061 and National Natural Science Foundation of China (82273635).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
All participants provided written informed consent, and the study was approved by the ethics committee of the Taicang Affiliated Hospital of Soochow University.
Open Research
DATA AVAILABILITY STATEMENT
The data included in this study are available on request from the corresponding author or the first author.




