Favorable outcome of open partial cystectomy for muscle-invasive squamous cell carcinoma of the bladder: A case report and literature review
Abstract
Key Clinical Message
The “gold standard” treatment for Squamous cell carcinoma (SCC) is radical cystectomy and different management approaches that combine chemotherapy and radiation in a neoadjuvant or adjuvant setting have been attempted with varying degrees of effectiveness. For certain individuals, partial cystectomy offers sufficient local control for muscle-invasive bladder cancer. Lifelong follow-up with cystoscopy is advised due to the possibility of potentially fatal late recurrence.
Squamous cell carcinoma (SCC) of the bladder is a rare urologic malignancy that is estimated to affect 3%–5% of the bladder cases. SCC of the bladder remains the most common subtype throughout Africa. Most of the literatures focused on the management of Urothelial carcinoma (UC), with fewer discussions on SCC management. UC typically presents with painless hematuria, whereas SCC presents with painful hematuria, bladder mass, and necroturia. SCC is mostly radioresistant and does not respond to chemotherapy. The mainstay treatment is partial cystectomy or radical cystectomy, which can be performed through open surgery or laparoscopic or robot-assisted approaches, all of which have acceptable results. We report a patient with a favorable outcome following partial cystectomy who was managed by open surgery. At the 12-month follow-up, the patient remained asymptomatic with good surgical outcomes.
1 INTRODUCTION
Squamous cell carcinoma (SCC) of the bladder is a rare urologic malignancy estimated to affect 3%–5% of the bladder cases.1 The most common histological subtype of bladder cancer in developed countries is Urothelial carcinoma (UC).2 SCC of the bladder remains the most common subtype throughout Africa.3,4 Most of the literature has focused on UC management of the bladder,5 with fewer discussions on SCC management. Urologists and surgeons practicing in Africa face the challenge of managing bladder cancer of the SCC subtype, which presents differently from UC.5 UC is associated with both smoking and working in the dye industry. On the other hand, SCC occurs in countries with a high schistosomiasis burden.4 UC is common in more industrialized countries, whereas SCC is more common in less industrialized countries.5 UC typically presents with painless haematuria, whereas SCC presents with painful hematuria, bladder mass, and necroturia.5 Like most urological diseases in developing countries, SCC presents late to the urologist, when the disease has advanced to the stage of muscular invasion.5 SCC is mostly radioresistant and does not respond to chemotherapy.
The mainstay treatment is partial cystectomy or radical cystectomy, which can be performed through open surgery or laparoscopic or robot-assisted approaches, all of which are techniques with almost acceptable outcomes.6
Solitary tumors without concomitant carcinoma in situ (CIS) that can be resected with 1–2 cm margins in a normally functioning bladder should be considered for partial cystectomy. Random bladder and prostate biopsies can be performed in addition to the standard work-up.6 We report a patient with a favorable surgical outcome following partial cystectomy and 12 months of follow-up.
2 CASE HISTORY
We report the case of a 32-year-old nulliparous woman who had hematuria and dysuria for 3 months. The patient complained of progressive, painful urination that was accompanied by necroturia. She also reported irritative lower urinary tract symptoms, with urgency being the most bothersome symptom, which was associated with initial hematuria and later progressed to total hematuria. Her condition was associated with on- and off-heartbeat palpitations, dull headache, and dizziness.
The patient visited several medical facilities and was frequently diagnosed with chronic recurrent cystitis. For almost 3 months, she has been switching to several antibiotics with no improvement. The patient did not report any history of smoking or exposure to chemical materials; however, she reported a positive history of schistosomiasis infections during her young age while working in rice plantations.
On physical examination, she had pale conjunctiva, not jaundiced, not cyanosed, and her vital signs were normal. An abdominal examination revealed normal findings. Blood workups revealed moderate anemia of 9 g/dL and was transfused 2 units of blood products before surgery and controlled hemoglobin was 11.8 g/dL. Renal, liver function tests and coagulation profiles were all within the normal range. Urinalysis revealed positive occult blood in addition to red blood cells and the presence of leukocytes. We collected a morning urine bacteriological culture for drug susceptibility analysis after we identified a urinary tract infection.
3 INVESTIGATIONS AND TREATMENT
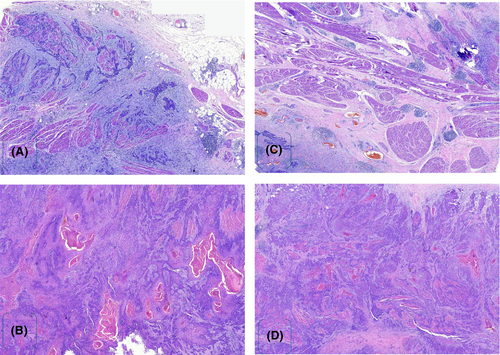
Kidney, ureter and bladder ultrasonography revealed a bladder mass at the right anterolateral wall of the bladder. Metastatic work-up, such as a thoraco-abdominopelvic CT scan, revealed normal findings. After controlling the infection with antibiotics, we performed a cystoscopy. Through cystoscopy, an intact urethra sphincter and a normal bladder neck with mild trabeculations were observed. A solid ulcerative mass was located at the right anterolateral bladder wall, and examination under anesthesia suggested T2aNxMx. Approximately three tissue samples were taken and sent for histopathology, which revealed an ulcerated transition epithelium with infiltrative tumor growing in keratin nests, suggesting grade 1 bladder SCC (Figure 1A,B).

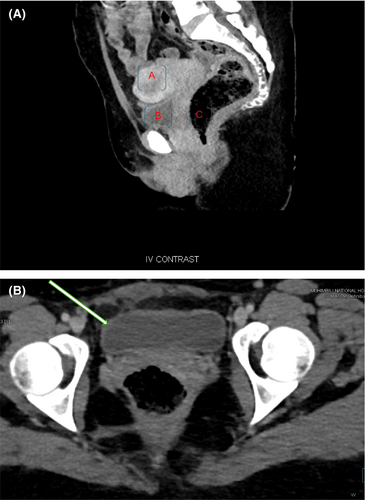
Abdominal pelvic T2-weighted MR image revealed an ulcerated bladder mass at the right anterolateral wall of approximately 2 × 3 cm with no evidence of lymphadenopathy (Figure 2A–C). After the relevant investigations were completed, Patient was planned for cystectomy with the primary objective of orthotopic neobladder surgery.

Through a median extended incision, the skin was opened in layers through an intraperitoneal approach. Dissection was carried out through the fascia, followed by opening of the peritoneum and development of the Retzius space. The patient had a normal bowel with no evidence of intraperitoneal organ metastasis. The colon was mobilized on each side along the white line of Told, to expose the retroperitoneum. The ureters were identified and swept medially. The pelvic organs were inspected, and no evidence of metastatic invasion seen (Figure 3).
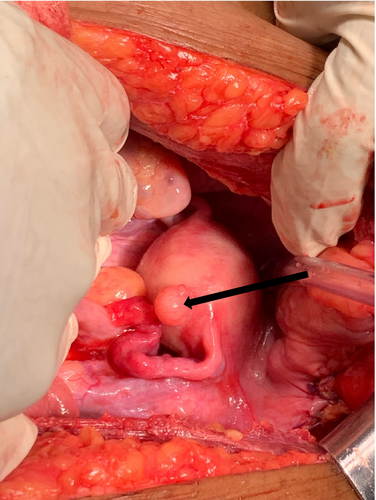
The surgical team was confident that there was no sign of tumor spread; therefore, we decided to perform a partial cystectomy. The bladder was identified and opened away from the tumor site, which was on the left anterior bladder wall. No stones or foreign bodies were observed in the bladder. An intravesical tumor with an exophytic growth of approximately 4.0 × 3.0 cm in diameter was identified at the right anterolateral wall of the bladder (Figure 4). The tumor was excised 2 cm from its edge, reaching deep into the muscular layer (Figure 5A,B). In ideal settings, intraoperative frozen sections can be sent to confirm the absence of microscopic disease at the margin; however, for our patient, this was not possible due to limited resource availability.
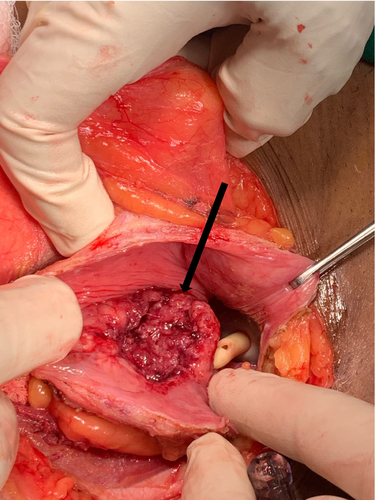
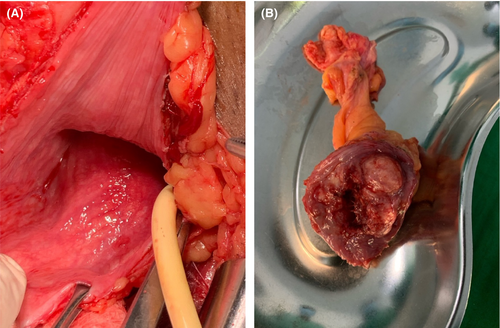
The bladder was closed in three layers, which included a mucosal, seromuscular, and adventitial layer, and then filled it to confirm watertight closure by using a mixture of saline and povidone (Figure 6). Dextrose 5% was used to irrigate the abdomen to cause lysis of the remaining tumor cells. A Foley catheter was left in situ for 21 days with no abdominal drainage placement. Approximately 70 mL of blood was lost. The wound was closed with interrupted 3/0 absorbable sutures.
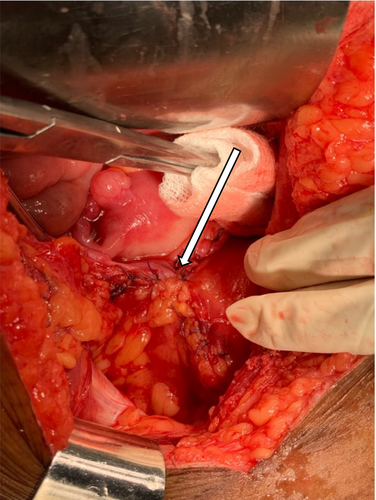
4 CONCLUSION AND RESULTS
Postoperative histological analysis revealed infiltrative neoplasia composed of pleomorphic cells with abundant eosinophilic cytoplasm. The tumor mass formed atypical keratin pearls involving all layers of the bladder with no vascular invasion and 3 cm negative surgical resection margins (Figure 7A–D). Postoperatively, there were no complications, the patient remained in the ward for 7 days, the wound healed, and the patient was discharged on the 8th day. The patient was instructed to return after 21 days for urethral catheter removal. She was followed for 12 months, and control cystoscopy revealed normal bladder wall mucosa with an adequate bladder capacity ranging from 350 to 400 mL, with no evidence of residual tumor seen. After 6 months a control CT scan showed normal pelvic wall organs with no evidence of recurrent metastasis (Figure 8A,B).
Patients with bladder SCC have a shorter survival period and a greater recurrence rate. Improving patient prognosis requires early diagnosis and treatment. Individualized treatment regimens should be devised based on the tumor's stage and grade, tumor type, size, presence or absence of metastases, and overall health. It is crucial that future research initiatives should prioritize the early diagnosis of squamous cell malignancies because bladder SCC typically manifests at a late stage and has a dismal prognosis. The “gold standard” treatment for SCC is radical cystectomy, and different management approaches that combine chemotherapy and radiation in a neoadjuvant or adjuvant setting have been attempted with varying degrees of effectiveness. For certain individuals, partial cystectomy offers sufficient local control for muscle-invasive bladder cancer. Lifelong follow-up with cystoscopy is advised due to the possibility of potentially fatal late recurrence.
5 DISCUSSION
Bladder cancer ranks as the tenth most common cancer worldwide according to 2020 Global Cancer Statistics. Worldwide, bladder cancer causes death of approximately 200,000 people annually. Women are more likely to develop SCC, which may be related to their propensity for cystitis and recurrent UTIs.7 For men, the incidence rate is currently either steady or declining, but for women, it is rising annually.7 SCC is the most common histological type of non-UC of the bladder. Based on these findings, up to 85% of SCC cases in Africa are still primarily linked to schistosomiasis infection. However, smoking, industrialization, and the management of schistosomiasis all have an impact on the evolving pattern of SCC.8
The percentages of SCC and UC vary significantly between African nations. In sub-Saharan countries, specifically in Tanzania, areas near inland freshwater lakes continue to have higher SCC levels than locations farther away.9,5 Since UC with squamous differentiation is far more prevalent than pure SCC, it is vital to distinguish between the two. SCC linked to schistosomiasis is referred to as bilharzial SCC, while SCC unrelated to the illness is referred to as non-bilharzial SCC.1 Schistosoma infestation is a risk factor for bilharzia, while non-bilharzia SCC risk factors include any trauma or insult that causes chronic inflammation, such as a history of spinal cord injury that results in neurogenic bladder and catheter dependence, recurrent UTIs, bladder calculi, cigarette smoking, or previous treatments such as intravesical BCG, pelvic radiation, and cyclophosphamide.1
Pathology revealed that SCC tends to be focally located as an ulcerative and nodular mass in the fundus of the bladder. The mass is usually larger than 3 cm in size at first presentation.10 At the time of initial presentation, SCC tends to be muscle invasive in 80% of cases.5
In contrast, UCs tend to be multifocal, small and papillary in shape with little or no muscle invasion at first presentation.11 SCC also has a lower grade, usually Grade 1, when patients are first seen in the hospital.11 UC, on the other hand, tends to be Grade 2 or 3 at first presentation. UC also spreads early to the lymph nodes, whereas SCC spreads late, possibly due to fibrosis of the lymphatic channels caused by Schistosoma eggs.5,11 Lymph node spread is observed in only 2%–10% of SCC patients.11
The majority of SCC patients die within 1–3 years of diagnosis, making it a disease with a poor prognosis.12 Due to the small number of clinical patients, there is a dearth of randomized prospective data to guide clinical treatment.
The main treatment for SCC is PC or radical cystectomy, which can be carried out using a laparoscopic or robot-assisted technique with satisfactory results. SCC is primarily radioresistant and does not respond to chemotherapy.6 Solitary tumors without concomitant CIS that can be resected with 1–2 cm margins in a normally functioning bladder should be considered for partial cystectomy. However, the morbidity associated with PC is significantly lower when compared to documented complications following RC.
According to a review by Shabsigh et al., there were 64% overall complications and 13% severe complications among 1142 patients who underwent RC.13,6 Consequently, performing PC is a suitable substitute for RC in patients who are carefully selected and unable to tolerate the associated morbidity. In our patient, no evidence of cancerous growth had spread to nearby organs or beyond the bladder during the surgical procedure.
Additionally, the patient was young and of reproductive age, making PC a suitable choice for her to avoid the morbid complications that radical cystectomy could cause in the future. Studies examining the overall survival of patients with squamous cell muscle invasive bladder cancer following partial cystectomy are scarcer than those involving UC patients. Kassouf et al. studied 37 patients who underwent PC for urothelial muscle invasive bladder cancer, and none of them had multifocal disease or CIS.14
AUTHOR CONTRIBUTIONS
Charles John Nhungo: Conceptualization; investigation; supervision; writing – original draft; writing – review and editing. Joseph Martin Lori: Writing – original draft. John Mugisha Kashaija: Conceptualization; data curation; formal analysis; investigation. SSirili Aloyce Harya: Supervision; writing – original draft. Rachel Kataraia: Investigation; supervision. Advera Ngaiza: Investigation; supervision. Obadia Venance Nyongole: Data curation; supervision; validation; writing – review and editing. Charles Mkony: Data curation; formal analysis; supervision; writing – review and editing.
ACKNOWLEDGMENTS
We would like to thank the whole urological team for the total support of our patient recovery.
FUNDING INFORMATION
There was no funding concerning this article.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
ETHICS STATEMENT
This case report study was exempt from ethical approval at our institution, as this paper reports a single case that emerged during normal surgical practice.
CONSENT
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal upon request.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




