Pressure-Wire Guided Hybrid Branch Pulmonary Artery Band Placement for Palliation of Single Ventricle and Critical Congenital Cardiac Lesions
ABSTRACT
Background
Historically, bilateral pulmonary artery band (PAB) placement was guided by pulmonary blood flow surrogates including systemic blood pressure (BP) and arterial saturation. These metrics alone may result in suboptimal bPAB placement. We perform a hybrid PAB (hPAB) procedure employing pressure-wire assessment to evaluate pulmonary hemodynamics and guide the procedure.
Methods
Single-center retrospective study of consecutive patients that underwent hybrid bPAB procedure between August 2015 and May 2022. Procedures involved main pulmonary artery (PA) angiography and selective PA pressure-wire assessment. Aortic pressure and arterial saturation were recorded.
Results
Twenty-three patients underwent hPAB procedure. Median procedure time was 190 min [range 122−480 min]. Ten patients underwent 15 total adjustments to a PA band, 6 (40%) based solely on pressure assessment with inappropriately low and non-pulsatile pressures. Five patients underwent 13 PA interventions after hPAB, 12 (92%) were transcatheter and 1 (8%) operative. Two patients underwent transcatheter angioplasty of a branch PA band in the interstage period while 4 underwent a total of 10 catheter-based PA interventions after the subsequent operation, at a median of 7 days [range 7−270 days] post-operatively. Two patients underwent PA stent implant. Branch PA Z-scores were normal at birth and remained normal at most recent follow-up for survivors (46 months), with final PA symmetry (smaller/larger PA area × 100) of 91% ± 9%.
Conclusions
Pressure-wire data identified more patients with overly restrictive bands during hybrid bPAB procedures than traditional markers alone. Many survivors required future PA intervention, and all demonstrated appropriate bilateral PA growth and symmetry.
Abbreviations
-
- AVC
-
- atrioventricular canal
-
- bPA
-
- bilateral pulmonary artery
-
- DBP
-
- diastolic blood pressure
-
- DORV
-
- double-outlet right ventricle
-
- ECMO
-
- extracorporeal membrane oxygenation
-
- HLHS
-
- hypoplastic left heart syndrome
-
- hPAB
-
- hybrid branch pulmonary arterial band
-
- HRHS
-
- hypoplastic right heart syndrome
-
- IAA
-
- interrupted aortic arch
-
- IC
-
- interventional cardiology
-
- IVH
-
- intraventricular hemorrhage
-
- LPA
-
- left pulmonary artery
-
- LV
-
- left ventricle
-
- MAP
-
- mean arterial pressure
-
- PA
-
- pulmonary artery
-
- PAB
-
- pulmonary artery band
-
- RPA
-
- right pulmonary artery
-
- SBP
-
- systolic blood pressure
-
- SD
-
- standard deviation
-
- SpO2
-
- arterial oxygen saturation
-
- VSD
-
- ventricular septal defect
1 Introduction
Bilateral branch pulmonary artery band (PAB) placement can be used as an early palliation technique for neonates with complex single or biventricular circulation [1, 2]. The procedure modulates pulmonary blood flow in patients at risk for pulmonary overcirculation in whom a main PAB is contraindicated or as part of the “Stage 1 hybrid” for infants with single ventricle physiology [3-5]. Determining the appropriate degree of band restriction is customarily assessed by visual inspection as well as surrogate markers of systemic and pulmonary blood flow including changes in systemic blood pressure (BP) and arterial oxygen saturation [6]. This traditional approach may be sub-optimal, as reliance on overall measures of systemic and pulmonary blood flow may result in asymmetric pulmonary blood flow, with the presence of either an overly restrictive or inadequately restrictive unilateral PAB. In this arrangement, even if only for weeks or months, sequelae include the risk of adverse distal pulmonary vascular remodeling or the development of subsequent pulmonary arterial stenoses that require intervention [7-9].
At our institution, bilateral PAB has been used for immediate stabilization in a subset of complex patients with ductal-dependent systemic blood flow and single or biventricular circulation who were in shock, had end-organ compromise, extreme prematurity, or for whom cardiopulmonary bypass (CPB) was strongly contraindicated (e.g., significant cerebral hemorrhage). The bilateral PAB strategy was used as a short- to medium-term bridge until single ventricle palliation or biventricular repair could be performed. To optimize post-operative and long-term outcomes after bilateral PAB placement, we perform hybrid branch pulmonary artery banding (hPAB) procedures with intraoperative pulmonary artery (PA) angiography and pressure-wire assessment.
We reviewed all patients undergoing hPAB procedures at our institution with the primary aim of evaluating the impact of this approach on intraoperative PAB revision as well as PA size and symmetry. We also evaluate the impact on interstage and post-palliation PA reinterventions.
2 Patients and Methods
We performed a retrospective review of all patients who underwent a hPAB procedure at our institution between August 2015 and May 2022. There were no exclusion criteria. Demographic variables, cardiac diagnoses, and comorbid conditions were recorded. Given our preference to perform a traditional Stage 1 Norwood or neonatal complete repair, hPAB procedures were typically performed as a temporizing measure in patients with contraindications to CPB (e.g., grade 3 intraventricular hemorrhage) or multiple comorbidities (e.g., very low birth weight, multiorgan dysfunction) deemed to impair surgical candidacy. The Cincinnati Children's Hospital Medical Center Institutional Review Board approved the study with a waiver of informed consent.
2.1 hPAB Procedural Technique
The bilateral PAB technique has been previously described, with an excellent technical description by Zampi et al. [10-12]. All but one case (40% O2) was performed in room air. To summarize, GoreTex bands were placed around each branch PA off CPB at a starting circumference of 7 mm regardless of size. After initial application of the bilateral PAB, a sheath was placed directly into the main PA, secured via a purse string suture and snared to allow fixation of the sheath. The interventional team then proceeded with branch PAB assessment, first with a main PA angiogram. A PAB was adjusted if clinical parameters (e.g., systemic saturation/BP and/or angiography) were out-of-range; repeat clinical and angiographic assessment was performed in those cases. Once angiography and clinical parameters were in-range, pressure-wire assessment of each branch PA was then performed in all patients. Given the narrow diameter of the branch PAB, we favor pressure-wire assessment over the use of larger microcatheters, which may obstruct flow and affect pressure readings. Specifically, a Glidecath catheter with Bentson-Hanafee-Wilson 1 distal curvature (Terumo Corp, Tokyo, Japan) was advanced to the main PA and utilized to direct a Pressure Wire X (Abbott, Lake County, IL USA) wire into each branch PA, with the sensor approximately 1−2 cm distal to the band and in the main branch PA lumen (Figure 1). The pressure wire was calibrated per manufacturer instructions. Goal branch PA mean pressures were < 20 mmHg with PA pulse pressure of 4−6 mmHg [12]. The PABs were secured to the PA adventitia in two locations, using 7−0 Prolene, to prevent distal migration.
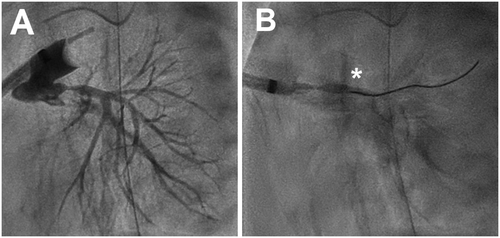
Indications to adjust a branch PAB were based on a non-algorithmic assessment of absolute and change from baseline in systemic arterial saturations and PO2, qualitative angiographic appearance of the banded branch PAs, and absolute and relative bilateral PA mean and pulse pressure. Regarding angiography, bands were considered appropriately restrictive if there was a visible and narrow (~2−3 mm diameter) waist at the site of application with relatively equal distribution of contrast and subsequent clearance through both PAs. An aforementioned vascular clip was removed for an overly restrictive band or an additional clip was applied if inadequate flow restriction. If adjustment of one or both bands was performed, both branch PAs were reassessed with angiography and pressure-wire measurement (Figure 2).
2.2 Concomitant and Subsequent Operative Procedures
Additional procedures performed at the time of hPAB were abstracted. These procedures included balloon atrial septostomy (BAS) and patent ductus arteriosus (PDA) stenting. Pulmonary arterioplasty was typically performed as part of the hPAB debanding procedure. For patients following a single ventricle pathway, a modified Stage 1 Norwood procedure was subsequently performed, which included PA debanding and augmentation with placement of either an RV-PA conduit (aka “Sano”) or Blalock-Taussig-Thomas (BTT) shunt. These patients typically underwent bidirectional Glenn (BDG) procedure at the traditional age following the modified Stage 1 Norwood operation. For patients with biventricular circulation, PA debanding and augmentation was performed contemporaneously with definitive biventricular repair.
2.3 Hemodynamics and Measurement of Pulmonary Arterial Growth
All catheterizations after the hPAB procedure were reviewed for all patients and hemodynamic data were recorded. With bilateral PAB in place, branch PA size was measured by angiography immediately distal to the band. As the PA band was typically immediately adjacent to a lobar branching point (e.g., right upper and intermediate PA), each segmental RPA diameter was measured, and values summated to calculate a composite branch PA measure. After branch PA debanding, branch PA diameter was measured immediately proximal to hilar branching by angiography, when obtained, and echocardiography. Branch PA measures are reported as both raw data and indexed to body surface area (BSA). In addition, post-hPAB procedure echocardiographic data were obtained, including spectral Doppler gradients and branch PA measures after debanding.
The Nakata index was calculated at hPAB procedure, after debanding, and most recent follow-up using the previously described formula [1, 2]. We measured branch PA Z-score and PA symmetry, defined as the ratio of the smaller to larger branch PA areas, expressed as a percentage.
2.4 Subsequent PA Interventions
All transcatheter and operative PA interventions performed on all hPAB patients were abstracted. Operative PA intervention was defined as any surgical procedure that involved pulmonary arterioplasty. As above, pulmonary arterioplasty is typically performed at the time of debanding and was not counted as a reintervention. Catheter-based interventions included PA balloon angioplasty with or without stent implantation.
2.5 Statistical Analysis
Statistical analysis was conducted using JMP (JMP Statistical Discovery LLC, Cary, NC). Standard descriptive statistics were performed. The paired t test was used to compare continuous variables. A two-sample proportion test was used to compare proportional variables. Time-to-event outcomes (freedom from PA intervention) were assessed with Kaplan−Meier survival estimates (log-rank test for difference between strata, p < 0.05).
3 Results
Twenty-three patients underwent hPAB during the study period. Baseline demographics, cardiac diagnoses, and comorbid conditions for hPAB patients are shown in Table 1. Median follow-up in patients who survived to the next planned operation was 46 [range 12−103] months.
| Age at hPAB (days) | 5 [3−12] |
| Gestational age (weeks) | 37.0 [31.3−39.2] |
| Prematurity (< 37 weeks) | 9 (39%) |
| Birth weight (kg) | 2.6 [1−4.2] |
| Female sex | 12 (52%) |
| Prenatal diagnosis | 18 (78%) |
| Diagnosis | |
| Biventricular lesions | 6 (26%) |
| IAA/VSD | 2 |
| Truncus arteriosus | 1 |
| Truncus/Common AVC | 1 |
| DORV | 1 |
| Structurally normal with severe LV dysfunction | 1 |
| Univentricular lesions | 17 (74%) |
| HLHS | 15 |
| HRHS | 2 |
| Genetic syndrome | 6 (26%) |
| Non-cardiac anomalya | 12 (52%) |
| Gastrointestinal | 7 (30%) |
| Genitourinary | 4 (17%) |
| Neurological | 7 (30%) |
| Stroke | 2 (29%) |
| IVH | 2 (29%) |
| Respiratory | 3 (13%) |
| Musculoskeletal | 5 (22%) |
- Note: Data presented as n (frequency) or median [range] or [IQR].
- Abbreviations: AVC = atrioventricular canal, DORV = double-outlet right ventricle, hPAB = hybrid branch pulmonary arterial band, HLHS = hypoplastic left heart syndrome, HRHS = hypoplastic right heart syndrome, IAA = interrupted aortic arch, IVH = intraventricular hemorrhage, LV = left ventricle, SD = standard deviation, VSD = ventricular septal defect.
- a Frequencies exceed 100% as some patients had more than one non-cardiac anomaly.
Table 2 summarizes details of the hPAB procedure. Patients underwent hPAB at a median age of 5 days [range 3−12]. Median total procedural duration for the entire cohort was 190 min, approximately 25 min longer for patients who underwent revision. Time from procedure start to interventional cardiology involvement was a median of 58 min. Five patients had concomitant ductal stenting or BAS, which were performed after application of the hPAB, while seven underwent BAS as a separate procedure, 4 (17%) before and 3 (13%) after.
| All patients (N = 23) | Revision (N = 10) | No revision (N = 13) | |
|---|---|---|---|
| Age (days) | 5 [3−12] | 7 [3−19] | 3 [1−22] |
| Weight at procedure (kg) | 2.7 [1−4.3] | 2.4 [1−4.3] | 2.7 [1.3−3.6] |
| Body surface area (m2) | 0.18 [0.1−0.24] | 0.16 [0.1−0.24] | 0.19 [0.11−0.23] |
| Other procedures | 5 (22%) | 2 (20%) | 3 (23%) |
| Ductus arteriosus stent | 3 (13%) | 1 (10%) | 2 (15%) |
| Balloon atrial septostomy | 2 (9%) | 1 (10%) | 1 (8%) |
| Pre-procedure vasopressors | 12 (52%) | 5 (50%) | 7 (58%) |
| Pre-procedure mechanical ventilation | 16 (70%) | 10 (100%) | 6 (46%) |
| Baseline hemoglobin (mg/dL) | 14.6 [12.5−19] | 14.6 [12.7−18.4] | 14.6 [12.5−19] |
| Total procedural duration (min) | 190 [122−480] | 199 [149−266] | 175 [122−480] |
| Time from start to IC involvement (min) | 58 [40−207] | 62 [51−87] | 56 [40−207] |
| bPA revision, N | 10 | ||
| Only RPA | 3 (30%) | ||
| Only LPA | 5 (50%) | ||
| Both PAs | 2 (20%) | ||
| Revision indications, N | 15 | ||
| Angiographic appearance ± pressure | 9 (60%) | ||
| PA pressure only | 6 (40%) | ||
| Angiography-based revision systemic saturation (%) | 66 [52−74] | ||
| Pressure-based revision systemic saturation (%) | 82 [74−86] | ||
| Angiography-based revision mean arterial pressure (mmHg) | 61 [54−72] | ||
| Pressure-based revision mean arterial pressure (mmHg) | 56 [38−70] | ||
| Pressure-based revision mean bPA pressure (mmHg) | |||
| RPA | 12 [12−13] | ||
| LPA | 11.5 [6−13] | ||
| Pressure-based revision bPA pressure pulsatility (mmHg) | |||
| RPA | 2 [1−2] | ||
| LPA | 1 [0−2] | ||
| Final systemic saturation (%) | 84 [78 – 88] | 84.5 [81−88] | 84 [78−88] |
| Final mean arterial pressure (mmHg) | 52 [38 – 68] | 53 [38−68] | 52 [40−66] |
| Final mean PA pressure (mmHg) | |||
| RPA | 18 [14−21] | 18 [14−21] | 18 [14−21] |
| LPA | 17 [14−19] | 17.5 [14−19] | 16 [14−19] |
| Final bPA pressure pulsatility (mmHg) | |||
| RPA | 4 [4−6] | 4 [4−5] | 4 [4−6] |
| LPA | 4 [4−6] | 4 [4−5] | 4.5 [4−6] |
- Note: Data presented as N (frequency) or median [range].
- Abbreviations: bPA = bilateral pulmonary artery, DBP = diastolic blood pressure, ECMO = extracorporeal membrane oxygenation, IC = interventional cardiology, LPA = left pulmonary artery, MAP = mean arterial pressure, PA = pulmonary artery, RPA = right pulmonary artery, SBP = systolic blood pressure, SpO2 = arterial oxygen saturation.
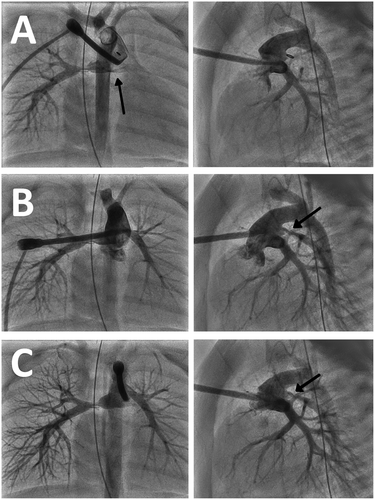
Intraoperative PA band adjustment was performed in 10 patients (43%). Among those 10 patients, a total of 15 branch PAB adjustments were performed, five on the RPA and 10 on the LPA. All but one revision was performed for an overly restrictive band, with one RPA band tightened based on angiography. Accounting for the 15 adjustments, two patients underwent a single adjustment of both bands and six patients underwent a single adjustment of only one PA band (three each on the RPA and LPA). Two other patients underwent multiple adjustments—2 in one, 3 in the other—of the LPA band. In both these cases the first adjustments were based on angiography, with the final adjustment based on pressure data. Among those 15 adjustments, 9 (60%) were based primarily on hypoxemia and angiographic appearance while 6 (40%) were based predominantly on out-of-range pressure measures that demonstrated minimal pulsatility (i.e., < 3 mmHg) and/or mean PA pressure ≤ 14 mmHg with qualitatively appropriate angiographic appearance and oxygen saturation. Bands were in place for a median of 25 days [range 1−239] before either surgical removal or patient death.
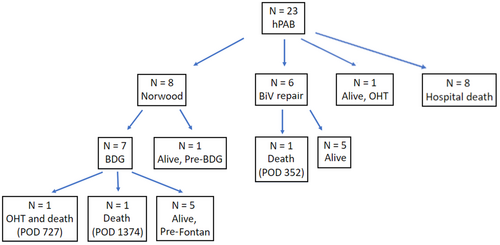
Figure 3 displays the ultimate outcomes of this cohort. Eight patients died before a subsequent cardiac operation, and one proceeded directly to cardiac transplantation. The remaining 14 underwent the subsequent planned operation, 8 of which were conversions to a surgical Stage 1 Norwood at a median age 22.5 days [range 6−85]. Of those, seven went on to a BDG at a median age of 4.2 months [range 3.2−7.1], with one patient interstage at the time of data analysis. The other six underwent biventricular repair at a median age of 3.8 months [range 1−8.1].
3.1 Hemodynamics
Table 2 includes procedural hemodynamics at the hPAB procedure. Median final PA pressures in all patients were 18 mmHg [range 14−21] in the RPA and 17 mmHg [range 14−19] in the LPA with similar median RPA and LPA pulse pressures of 4 mmHg (range 4−6 mmHg). Among the adjustments for an overly restrictive band, median pre-revision RPA pressure was 12 mmHg [range 12−13] with pulse pressure of 2 mmHg [range 1−2] and median LPA pressure was 11.5 mmHg [range 6−14] with pulse pressure of 1 mmHg [range 0−2]. Among the nine patients who underwent PAB revision for hypoxemia or angiographic appearance, arterial oxygen saturation increased from a nadir of 66% ± 9% after initial application of the bilateral PAB to 86% ± 8% (p < 0.004) after adjustment. Regarding the six who underwent revision based on out-of-range pressures, saturations did not significantly change (pre-revision 82% ± 3% and vs. post-revision 81% ± 2%).
3.2 Pulmonary Arterial Reinterventions
Five patients—1 single-ventricle and 4 two-ventricle—underwent a total of 13 procedures to intervene on the branch PAs after hPAB (Table 3). One underwent reintervention only in the interstage, three underwent reintervention only after the subsequent operation, and one underwent reinterventions both during the interstage and after repair. The five patients had a median of two reinterventions [range 1−4] each, at a median interval of 111 days [IQR 82−242] after hPAB procedure. Four patients had only transcatheter reinterventions while one had both transcatheter and surgical revisions.
| Patients who underwent PA reintervention | 5 (22%) |
| Before debanding | 2 (9%) |
| Post debandinga | 4 (17%) |
| Indications | |
| Elevated PA doppler gradient | 2 (40%) |
| Asymmetric lung perfusion | 2 (40%) |
| Ventricular dysfunction | 1 (20%) |
| Total PA reinterventions | 13 |
| Median reinterventions per patient | 2 [1−4] |
| Branch PA intervened upon | |
| RPA | 1 [1−2] |
| LPA | 3 [2−4] |
| Mode of reintervention | |
| Transcatheter | 12 (92%) |
| Balloon angioplasty only | 10 (83%) |
| Stent only | 1 (8%) |
| Both | 1 (8%) |
| Surgical | 1 (8%) |
| Duration hPAb to 1st reintervention (days) | 111 [47−396] |
- Note: Data presented as n (frequency) or median [range].
- Abbreviations: bPAB = bilateral pulmonary arterial band, hPAB = hybrid branch pulmonary arterial band, LPA = left pulmonary artery, PA = pulmonary artery, RPA = right pulmonary artery, SD = standard deviation.
- a one patient underwent PA intervention before and after debanding.
Of the 13 procedures, 12 were transcatheter and 1 was surgical. As above, 2 of the 12 transcatheter reinterventions were performed during the interstage, both involving angioplasty of a bPA band in the setting of hypoxemia related to somatic growth; neither had undergone PAB revision at the time of the hybrid procedure. One of those was in a single-ventricle patient, at 47 days post-hPAB, with RPA band angioplasty alone. The other was a biventricular patient, 142 days after hPAB, involving bilateral PAB angioplasty. The remaining 10 catheterizations were performed after debanding, all on patients with two-ventricle circulation. The single operative PA revision was performed on a patient with Taussig-Bing type DORV who had undergone DORV repair involving a LeCompte maneuver. He underwent repeat operation for LPA arterioplasty and transannular patch related to combined RV outflow and LPA obstructions; this patient went on to have 3 transcatheter bPA angioplasties of the LPA.
Of the total 46 bPAs banded in the cohort, 2 RPAs underwent reintervention after debanding, comprising 17% of the 12 survivors' RPAs. One intervention involved RPA angioplasty alone and the other an RPA stent implant. As for the LPA, 10 underwent reintervention, 90% of survivors' LPAs, with eight angioplasty-only and two LPA stent implants.
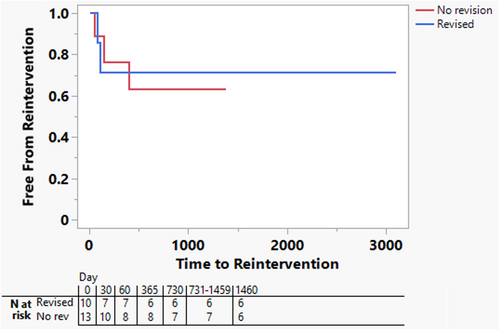
Freedom from PA reintervention is shown in Figure 4. Though sample sizes are limited, freedom from reintervention was similar among patients who underwent intraoperative revision and those who did not.
3.3 Pulmonary Arterial Size, Growth, and Symmetry
Table 4 outlines longitudinal PA measures for the cohort. Branch PA sizes were in the normal range at the time of the hPAB procedure with no significant difference between patients who underwent branch PAB revision and those who did not (Nakata 245 ± 65 vs. 237 ± 59 respectively, p = 0.02). PA parameters remained in the low-normal range at most recent follow-up, again with no significant differences between the revision and no revision patients (239 ± 26 vs. 259 ± 42 respectively, p = 0.03). Patients with single ventricle physiology had final PA measures that were slightly smaller than the biventricular patients, consistent with other published findings in single ventricle patients (225 ± 14 in single ventricle vs. 286 ± 26 in biventricular patients, p = 0.01) [13].
| RPA parameters | LPA parameters | Nakata index (mm2/m2) | |||
|---|---|---|---|---|---|
| RPA (mm) | Z score | LPA (mm) | Z score | ||
| At hPAB procedure (n = 23) | |||||
| Entire cohort (n = 23) | 4.8 ± 0.9 | 0.8 ± 0.7 | 4.4 ± 0.7 | 0.4 ± 0.6 | 241 ± 60 |
| bPAB revision (n = 10) | 4.7 ± 0.8 | 0.9 ± 0.7 | 4.4 ± 0.7 | 0.5 ± 0.7 | 245 ± 65 |
| Post debanding | |||||
| All debanded (n = 15) | 5.8 ± 0.8 | 0.4 ± 0.8 | 5.6 ± 0.9 | 0.3 ± 0.7 | 229 ± 49 |
| bPAB revision (n = 7) | 5.6 ± 0.9 | 0.4 ± 0.9 | 5.5 ± 1.1 | 0.4 ± 0.8 | 231 ± 56 |
| Most recent follow-upa | |||||
| Entire cohort (n = 15) | 8.9 ± 0.7 | 0.6 ± 0.5 | 8.2 ± 0.9 | 0.1 ± 0.6 | 249 ± 36 |
| bPAb revision (n = 7) | 9.1 ± 0.6 | 0.4 ± 0.5 | 8.4 ± 0.8 | 0.1 ± 0.4 | 239 ± 26 |
| PA growth | RPA (mm/year) | Z score change | LPA (mm/year) | Z score change | Nakata index (mm2/year/m2) |
| From hPAB procedure | 2.0 ± 1.4 | 0.1 ± 0.7 | 1.9 ± 1.3 | 0.0 ± 0.6 | 17.7 ± 52.2 |
| From debanding | 1.7 ± 1.4 | 0.3 ± 0.9 | 1.4 ± 1.3 | 0.1 ± 0.7 | 26.2 ± 54.2 |
- Note: Data presented as mean ± standard deviation.
- Abbreviation: bPAB = bilateral pulmonary arterial band, hPAB = hybrid pulmonary arterial band, PA = pulmonary artery, PAB = pulmonary arterial band, SD = standard deviation.
- a Patient excluded if most recent measurement < 180 days after hPAB procedure.
From a PA symmetry standpoint, symmetry was 91% ± 5% at completion of the hPAB procedure. Symmetry for the entire cohort was 91% ± 7% at most recent follow-up, with no significant differences based on circulation type (92% ± 5% single ventricle vs. 90% ± 11% two ventricles, p = 0.2) or performance of intra-operative band revision (91% ± 6% revised vs. 91% ± 9% no revised, p = 0.4). Central illustration 1
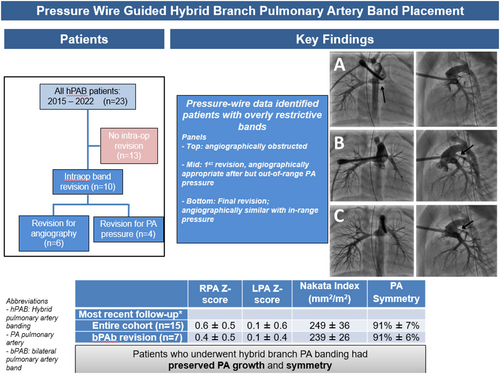
3.4 Final Status of Hpab Patients
Fifteen patients (65%) survived to repair/Stage 2, which was performed at a median age 127 days [range 31−242]. Eight proceeded to a univentricular palliation and underwent BDG, six underwent biventricular repair, and one received an OHT shortly after hPAB. All patients with univentricular circulation remained hospitalized after the hPAB procedure until the Stage 1 Norwood. Conversely, 2 of the patients with biventricular circulation were discharged after the hPAB while four remained hospitalized until repair. After the index operation, 14 patients were ultimately discharged (8 uni- and 6 biventricular), at a median age of 45 days [range 24–89].
Overall mortality was 48% (N = 11). Of the eight who died before the next planned operation, seven had univentricular circulation. The median age of interstage death was 11 days [range 4−60] in those with univentricular lesions (Table 5). Of the three who died after debanding, one had undergone biventricular repair, was discharged at 79 DOL, subsequently hospitalized on at 9 months of age for respiratory decompensation, and ultimately died at 11 months of age (352 days after the hPAB procedure) with in-home hospice. The second patient underwent BDG and was discharged at 49 days of age, developed significant ventricular dysfunction, and received an OHT (727 days after hPAB) after prolonged hospitalization; this patient died 161 days post-transplant. The final patient underwent BDG and was discharged on DOL 106; the patient later died at 3 years of age (3.9 years after hPAB procedure) after in-hospital cardiac arrest during an admission for pneumonia.
| Death before repair/stage 2 | 8 (36%) |
|---|---|
| Univentricular | 7 (88%) |
| Biventricular | 1 (12%) |
| Age at pre-repair/stage 2 death (days) | |
| Univentricular | 11 [4–60] |
| Biventricular | 37 [n/a] |
| Survival to repair/stage 2 | 15 (65%) |
| Univentricular | 9 (60%) |
| Biventricular | 6 (40%) |
| Age at repair/stage 2 (days) | 127 [31–242] |
| Major complication at repair/stage 2 | 2 (14%) |
| Death after repair/stage 2 | 3 (21%) |
| Univentricular | 2 (66%) |
| Biventricular | 1 (33%) |
| bPA reintervention after repair/stage 2 | 4 (17%) |
| Transcatheter only | 3 (75%) |
| Operative and transcatheter | 1 (25%) |
| Residual bPA stenosis after repair/stage 2 | 4 (27%) |
| RPA only | 0 |
| LPA only | 1 |
| Both PAs | 3 |
- Note: Data presented as N (frequency) or median [range].
- Abbreviations: bPA = bilateral pulmonary artery, LPA = left pulmonary artery, PA = pulmonary artery; RPA = right pulmonary artery, SD = standard deviation.
4 Discussion
This study describes procedural details and evaluates the impact of hPAB, specifically describing immediate intraoperative band revision and initial PA symmetry. We also describe long term PA growth/symmetry and reinterventions after band removal. Broadly, our cohort experience demonstrated that a significant number of patients undergo intraoperative adjustments of at least one branch PAB. Moreover, 6 of the 15 readjustments were performed solely for suboptimal pressure assessment that demonstrated low mean and minimally/non-pulsatile PA pressure waveforms. Further, the cohort on whom this technique was utilized demonstrated a high degree of PA symmetry, with normal PA growth long-term. Finally, a minority of patients underwent branch PA reintervention after debanding, which is consistent with prior studies [7-9].
Optimal bPA band resistance is important for interstage physiology and symmetric PA growth is important long-term, especially for single ventricle patients. Traditional markers used during application of PA bands, such as systemic saturation and BP, are effective measures of the overall pulmonary-to-systemic flow ratios. However, those indices are unable to provide meaningful and reliable information on pulmonary flow balance. As our data demonstrated, 6 of the 15 (40%) revisions were performed on patients in whom traditional parameters suggested appropriate bPA banding (e.g., changes in systemic oxygen saturation/mean arterial pressure and angiographic appearance), with the out-of-range pressure measures being the primary impetus to perform band revision. Thus, our experience suggests that pressure-wire assessment provided significant, clinically relevant information in a relatively high proportion of cases.
In addition to technical operative outcomes, appropriately restrictive bilateral PABs are important for optimal interstage physiology. One issue is that excess restriction may lead to clinically significant hypoxemia before the target age and weight for the next planned intervention [14]. This is especially relevant since a significant subset of bPAB patients, both in our experience and at other centers, are premature/low weight or have multiple comorbidities and may benefit from an extended interstage period until the subsequent operation [15]. Another nuance is that the duration of the branch PA bands is unpredictable in this cohort. For example, hemodynamic issues that led to hybrid bPAB placement (e.g., significant AV valve regurgitation or diminished ventricular function) may persist for weeks. In those cases, conversion to complete “hybrid” Stage 1 physiology may be indicated with PDA stent placement; appropriately restrictive and balanced bPA flow is especially important in these patients who necessitate longer duration for the bands.
Multiple studies have demonstrated that bPAB patients experience higher rates of PA reintervention compared to traditional palliation approaches. For example, PA reintervention rate have been described at 28%−31% in hybrid patients compared to 11% in the traditional Stage 1 group, with lower freedom from reintervention at 1 and 3 years, and intervention free-survival rate of only 20% at 5 years in the hybrid patients [7, 8]. Another study examined a cohort of HLHS patients undergoing a hybrid stage I procedure and noted that only 32.2% were free from PA intervention at 10 years [9]. Sample sizes in these and our study preclude precise comparisons, but our experience was largely similar.
Of those that had a PA reintervention, we noted a significant predominance of reinterventions on the LPA, similar to other reports [15-17]. Interestingly, three of the five patients who underwent reintervention had a biventricular repair. Studies have demonstrated that certain operations, particularly involving placement of an RV-PA conduit or RVOT enlargement, may lead to suboptimal alterations in branch PA anatomy necessitating intervention [18, 19].
Finally, all patients in our cohort, including those with intraoperative bPAB adjustment, demonstrated appropriate PA growth with normal symmetry following debanding regardless of repair type. In fact, final measures of both LPA and RPA were similar, if not superior, to other studies [13, 20]. Thus, our data suggest that this staged hybrid bPAB approach does not confer a significant long-term disadvantage compared to traditional treatment strategies.
4.1 Limitations
Ours is a retrospective review of one a single cohort, without a direct comparison, at a single center. Duration of follow-up (mean 25 months) is relatively short. Further, the heterogeneity of the cohort may independently influence short- and long-term outcomes independent of the effects of hPAB. And since hPAB is not widely performed, threshold pressures were developed a priori, and further study may suggest finer calibration of goal pressures. And though we evaluated PA reinterventions and growth, long-term quantitative branch PA flow data were limited in our cohort. Future studies comparing this technique with traditional branch PA band placement can help better understand the effect on subsequent pulmonary arterial interventions, measured branch PA flow symmetry, Fontan candidacy, and other clinical outcomes.
4.2 Conclusions
The pressure-wire assisted hPAB procedure is a useful technique that helps identify patients with overly restrictive bands that may not be identified by traditional clinical markers—BP and saturations—or angiography. These real-time data provide an opportunity for intraoperative adjustment with the goal of achieving symmetric PA flow.
Acknowledgments
The authors would like to acknowledge James A. Tweddell, MD who played a significant role in this technique at our institution.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




