Efficacy and Safety of Intravascular Lithotripsy in the Management of Underexpanded Stents: A Systematic Review and Meta-Analysis
ABSTRACT
Background
Stent underexpansion significantly heightens the risk of major adverse cardiac events (MACE), and available treatment options for this condition remain limited. Intravascular Lithotripsy (IVL) technology disrupts superficial and deep calcium by using localized pulsative sonic pressure waves, emerges as a promising tool for underexpanded stents.
Aims
This study examines the overall efficacy and safety of IVL, an until-now off-label modality, in the management of underexpanded stents.
Methods
Following PRISMA guidelines, we systematically explored PubMed, SCOPUS, and Cochrane databases up to April 30, 2024, for studies evaluating IVL's safety and efficacy in treating underexpanded stents. We gathered angiographic (QCA) and intracoronary imaging (OCT or IVUS) data, examining the stent's diameter stenosis (SDS), minimal lumen diameter (MLD), minimal stent area (MSA), and minimal lumen area (MLA) pre- and post-IVL application. Procedural success constituted the efficacy endpoint, while peri-procedural complications, in-hospital-30-days and long-term mortality, and MACE were safety endpoints.
Results
This meta-analysis comprised 23 studies including 819 patients and 837 treated lesions in underexpanded stent. The mean age was 71.7 ± 8.8 years, with an overall IVL procedural success rate of 92% [(95% confidence interval (CI): 88%–95%, I2 = 35%), while the in-hospital-30-days and long-term mortality incidence were 1% (95% CI: 1%–3%, I2 = 0%) and 4% (95% CI: 2%–6%, I2 = 0), respectively. The 30-day rates acute myocardial infarction and stroke were 1% [(95% CI: 0%–1%, I² = 0%), (95% CI: 0%–2%, I2 = 0%)] each. No need for short term target lesion revascularization (TLR) was observed while the long-term rates were 6% (95% CI: 3%–10%, I2 = 48%). There was a significant decrease in the SDS [Standardized Mean Difference (SMD): −3.57 (95% CI: −4.64 to –2.44%, I2 = 94%)] and increase in MSA (SMD: +1.98, 95% CI: 0.86–3.09, I2 = 93%) after IVL application. It was observed a significant increase in MLD (SMD: +2.68, 95% CI: 1.94–3.41, I2 = 90%) and in the MLA (SMD: +1.92, 95% CI: 1.46–2.38, I2 = 69%). Major procedural and device related complications were 2% (95% CI: 1%–5%, I2 = 0%) and 1% (95% CI: 0%–2%, I2 = 80%) respectively. Notably low rates were observed for stent thrombosis (1%, 95% CI: 0%–2%, I2 = 0%), dissections (1%, 95% CI: 1%–4%, I2 = 0%), perforations (1%, 95% CI: 1%–3%, I2 = 0%) and no-reflow (0%, 95% CI: 0%–46%, I2 = 0%).
Conclusions
IVL demonstrates promise as a safe and effective strategy for underexpanded stent treatment, characterized by low rates of periprocedural complications. Future prospective studies are now warranted to compare IVL to other lesion preparation strategies.
1 Introduction
Coronary artery calcification poses a significant challenge during percutaneous coronary interventions (PCIs) and is correlated with suboptimal stent expansion and malapposition, leading to higher rates of adverse cardiovascular events [1, 2]. While various modalities have been introduced to address this issue, the method of intravascular lithotripsy (IVL) has gained substantial attention for its potential to effectively modify and disrupt calcified lesions, thereby facilitating optimal stent deployment and improving clinical outcomes. IVL utilizes sonic pressure waves generated by an integrated balloon-based system to disrupt calcified plaques, promoting plaque modification and expansion, and subsequently facilitating stent apposition and expansion [3]. Previous studies have demonstrated promising outcomes with IVL in terms of lesion preparation, stent optimization, and procedural success rates, suggesting its potential as a viable solution for the challenging subset of patients with heavily calcified coronary lesions [3, 4]. However, the overall efficacy, safety profile, and clinical implications of IVL across various patient populations and lesion complexities remain subjects of ongoing investigation and debate.
In light of the growing interest and the need for a comprehensive evaluation of the existing evidence, we conducted a systematic review and meta-analysis to assess the overall efficacy and safety of IVL, an until-now off-label modality, in the management of underexpanded stents.
2 Methods
This analysis was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines [5] (Appendix S1: Figure 1 and Table 2). The protocol for this analysis was registered in the PROSPERO database with number CRD42024563473. Given that this was a meta-analysis of previously published data, no ethical approval was required.
2.1 Search Strategy
A systematic literature search was carried out up to March 30, 2024 using MEDLINE (Pubmed), Scopus and Cochrane Library without applying any language restrictions. A search strategy was developed using the following search algorithm: ([stent underexpansion] OR [in-stent restenosis] OR [stent thrombosis] OR [stent failure]) AND ([lithotripsy] OR [lithoplasty]). Reference lists from selected studies were carefully examined to identify any additional eligible studies.
2.2 Data Extraction and Eligibility Criteria
Initially, all records that were retrieved from electronic search were inserted to a reference managerce and duplicates were removed. Then, all titles and abstracts were screened for relevance by two independent and authors (M.S., S.S.). Full-text publications of articles that appeared relevant were subsequently examined for eligibility. Potential discrepancies were resolved by consultation with a third reviewer (N.K.). Prospective or retrospective studies evaluating the safety and efficacy of IVL in addressing resistant stent underexpansion were considered eligible for this analysis. To maintain a reasonable level of data inclusion while ensuring reliability, we excluded studies with fewer than five patients. This threshold was chosen to avoid excessive heterogeneity from single-case reports or very small cohorts while still incorporating valuable clinical data. A data extraction form was designed according to the Cochrane checklist of items (PICO—Patients, Interventions, Comparisons, Results), where data from eligible studies were inserted. The quality assessment of the eligible studies was performed using the modified Newcastle-Ottawa Scale (NOS) (Appendix S1), a tool that has been developed for the assessment of nonrandomized studies and is based on the evaluation of three quality parameters (patient selection, comparability, and outcome).
2.3 Definition of Endpoints
The prespecified primary efficacy outcomes were as follows: (1) 30-day and long-term all-cause mortality and (2) IVL procedural device success, which in most studies was defined as successful IVL delivery and stent expansion with a residual stenosis < 20%. The secondary safety outcomes included: (1) major procedural complications, including vessel perforation, dissection, slow/no flow phenomenon or periprocedural myocardial infraction, (2) MACEs at 30-day and long-term follow-up, defined as cardiac death, myocardial infarction or target vessel revascularization.
2.4 Angiographic Data
Pre- and postprocedural angiographic measurements derived by either quantitative coronary angiography (QCA) or intravascular imaging modalities, namely intravascular ultrasound (IVUS) and optical coherence tomography (OCT) were collected. In particular, the following parameters were obtained: lesion length, pre- and postprocedural reference vessel diameter, stent length, pre- and postprocedural minimal lumen diameter (MLD), pre- and postprocedural minimal stent area (MSA) and the pre- and postprocedural minimal lumen area (MLA). Where given, we decided to include angiographic data derived by either IVUS or OCT over QCA, taking into consideration the higher level of accuracy of these methods. The included studies provided preprocedural measurements at an initial stage of the procedure, before attempting any modification of the lesions, while postprocedural measurements referred to the final result after completing IVL and the last balloon dilatations of the lesion.
2.5 Data Synthesis and Statistical Analysis
The meta-analysis was conducted in R Statistical Software (version 4.2.2, Boston, MA, USA) using the packages “meta,” “metafor” and “metaprop.” A random effects model (DerSimonian and Laird method) was applied to estimate pooled primary and secondary outcome rates. All pooled estimates are provided with 95% confidence intervals (95% CIs). The weight of each study in the meta-analysis was estimated using the inverse variance method. The baseline characteristics were analyzed with descriptive statistics and are reported either as mean values with standard deviation or as median values and range. The heterogeneity across studies was assessed with the Cochrane's Q-test and the I2 index, with I2 < 25% indicating low, 25%–50% moderate, and > 50% high heterogeneity.
2.6 Quality and Risk of Bias Assessment
Study quality scores were ascertained using the modified Newcastle-Ottawa Scale (NOS) [6] for cohort studies. The NOS has been developed to assess the methodological quality of nonrandomized studies. Each study was assigned a maximum of four points for selection of the study population, two points for comparability, and three points for assessment of the outcome.
3 Results
3.1 Study Selection
The combined search yielded 447 unique publications, of which 85 full-text articles were retrieved and reviewed for eligibility for our analysis. Of these, 23 studies involving a total of 819 patients were ultimately included in our analysis [7-28]. The PRISMA flow diagram of our study selection is depicted in Appendix S1. All the studies included in the analysis were either retrospective studies or prospective observational cohorts. Regarding the follow-up duration, some studies provided data only about the immediate postinterventional period during the hospital stay, whereas it extended up to 16 months in others. The indication for the application of IVL was stent under-expansion due to high volume of calcium.
The mean age of the included population was 71.7 ± 8.8 years with the majority of patients being males. Full patient characteristics are presented in Table 1. The total number of the treated lesions was 894. The affected vessels were 13.3% (N = 62/465) the left main artery, 41.2% (N = 235/570) the left anterior descending artery, 14.4% (N = 81/563) the circumflex artery, 33.1% (N = 191/577) the right coronary artery and the vein graft in eight cases. Preprocedural examinations revealed a median lesion length at 24 mm. The median total procedural time was 89 min, the total fluoroscopy time was 26 min, the contrast volume during the procedure was 195 mL and a median number of 65 IVL pulses were used. In most cases, lesion preparation before IVL was performed using noncompliant balloons, while the use of cutting balloons, laser, or rotational atherectomy was rare. Predilation was attempted in 81.6% (382/468) of cases, while postdilation was performed in 76% (342/450). Intravascular imaging (IVUS or OCT) was utilized in 60.3% (262/434) of patients, with IVUS used in 41.4% (180/434) and OCT in 18.9% (82/434) of cases.
| Study | Design | Follow-up (months) | N patients | Age (mean) | CCS | UA | NSTEMI | STEMI | IVUS/OCT |
|---|---|---|---|---|---|---|---|---|---|
| J. Hinton et al. | Retrospective | 16.8 | 39 | 71 | |||||
| A. Ielasi et al. | Retrospective | 1 | 34 | 68.5 | 19 | 5 | 9 | 1 | 19 |
| W. Wańha et al. | Retrospective | 10.8 | 62 | 69 | 30 | 10 | 20 | 2 | 29 |
| A. Doost et al. | Retrospective | 1 | 8 | ||||||
| M. Forero et al. | Retrospective | 1 | 70 | 73 | 32 | 12 | 18 | 7 | |
| K. Yaginuma et al. | Retrospective | 7 | 6 | ||||||
| A. Gonzálvez-García et al. | Retrospective | 7 | 6 | ||||||
| Angelo Mastrangelo | Retrospective | 8.9 | 11 | 73.6 | 11 | 0 | 0 | 0 | 3 |
| Angelo Mastrangelo | Retrospective | 12.1 | 22 | 72.4 | 15 | 1 | 4 | 2 | 11 |
| H. Sinclair et al. | Retrospective | 1 | 12 | 8 | |||||
| T. Takahashi et al. | Retrospective | 17 | 12 | ||||||
| A. Thandra et al. | Retrospective | 6.6 | 7 | 6 | |||||
| V. Pham et al. | Retrospective | 1 | 25 | 72.2 | |||||
| S. Umapathy et al. | Prospective | 1 | 12 | 70 | |||||
| B. Honton et al. | Prospective | 12 | 47 | 72 | |||||
| J. Yeoh et al. | Prospective | 13 | 70.2 | ||||||
| F. Brunner et al. | Prospective | 4.5 | 6 | 75.5 | 4 | 2 | 0 | 0 | 3 |
| H. Jattari et al. | Prospective | 40 | 73.7 | ||||||
| P. Rola et al. | Prospective | 6 | 131 | 70.8 | 17 | 6 | 97 | 11 | |
| A. Aksoy et al. | Prospective | 1 | 71 | 76 | 33 | 11 | 10 | 1 | 35 |
| P. Dwivedi et al. | Prospective | 21 | 69.3 | 19 | 10 | ||||
| K. Yasumura et al. | Prospective | 1 | 56 | 69 | 46 | 10 | 56 | ||
| M. van Oort et al. | Retrospective | 12 | 101 | 71.2 | 44 | 20 | 28 | 9 | 58 |
- Abbreviations: CCS = chronic coronary syndrome, IVUS = intravascular ultrasound, N = number, NSTEMI = non-ST-elevation myocardial infarction, OCT = optical coherence tomography, UA = unstable angina.
3.2 In-Hospital/30-Days Outcomes
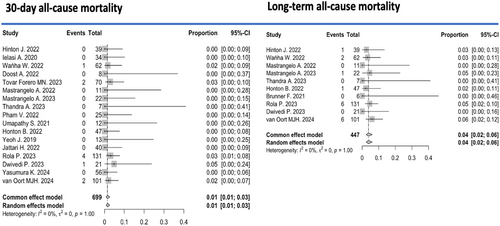
The pooled all-cause mortality rate during the periprocedural period (up to 30 days postprocedure) across 17 studies [7-11, 14, 17-21, 23, 24, 26-28] was 1% (95% CI: 0%–3%, I2 = 0) (Figure 1). Within the first 30 days after the intervention, the pooled incidence of acute myocardial infarction was 1% (95% CI: 0%–2%, I2 = 0%) among 711 patients, and the pooled incidence of stroke was 1% (95% CI: 0%–2%, I2 = 0%) among 318 patients (Appendix S1).
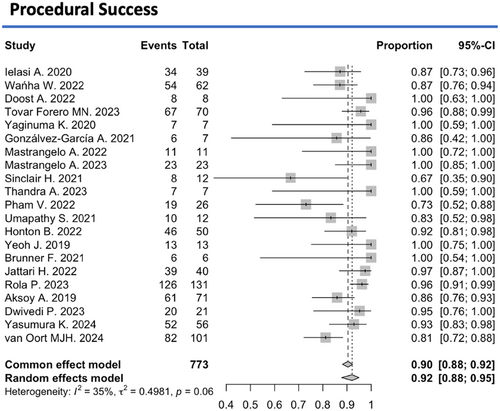
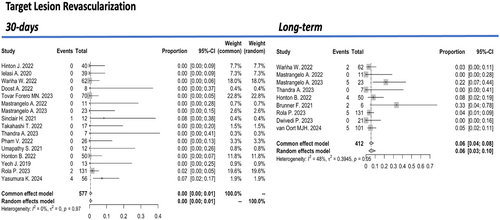
Successful IVL performance and adequate stent expansion were achieved in 92% (95% CI: 88%–95%, I2 = 35%) of 773 treated lesions (Figure 2). However, major procedural-complications occurred in 2% (95% CI: 1%–5%, I² = 0%) of patients and device-related complications in 1% (95% CI: 0%–2%, I2 = 80%) (Appendix S1). Specifically, coronary artery perforation occurred in 1% (95% CI: 0%–3%, I2 = 0%), dissection in 1% (95% CI: 0%–4%, I2 = 0%), slow flow or no reflow phenomena in 0% (95% CI: 0%–38%, I² = 0), and stent thrombosis in 1% (95% CI: 0%–2%, I2 = 0%) of treated lesions during the immediate postinterventional period. Target lesion revascularization (TLR) during the first 30 days after the procedure was 0% (95% CI: 0%–1%, I2 = 0%) (Figure 3).
3.3 Intravascular Imaging Alterations
Based on the intracoronary imaging measurements (IVUS, OCT), the mean minimal lumen area (MLA) was reported in six studies [9, 11, 15, 17, 27, 28], with a pre-IVL value of 2.9 mm² (95% CI: 2.4–3.4 mm2) and a post-IVL value of 6.7 mm2 (95% CI: 6.1–7.3 mm2, I2 = 66%), showing a standardized mean difference of +1.92 (95% CI: 1.46–2.38, I2 = 69%). The mean minimum luminal diameter (MLD), reported in 10 studies [8, 9, 11, 12, 14, 19, 22, 25, 27, 28], was 1.0 mm (95% CI: 0.8–1.2 mm, I2 = 87%) before the intervention and 2.5 mm (95% CI: 2.3–2.8 mm, I2 = 96%) after the intervention, indicating a standardized mean difference of +2.68 (95% CI: 1.94–3.41, I2 = 90%) (Figure 4).
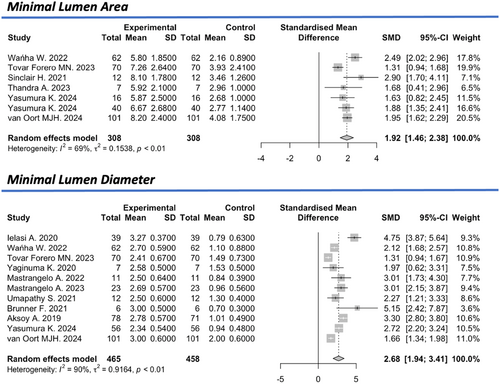
The mean minimum stent area (MSA), reported in five studies [7-9, 11, 21, 28], was 3.3 mm2 (95% CI: 2.82–3.94 mm2, I2 = 88%) before the intervention and 6.8 mm² (95% CI: 6.4–7.2 mm2, I2 = 63%) after the intervention, reflecting a significant standardized mean difference of +1.98 (95% CI: 0.86–3.09, I2 = 93%). The overall estimated preinterventional mean stent's diameter stenosis (SDS) derived by QCA was 66.9% (95% CI: 61.4–72.4%, I2 = 92%), which was reduced to 15% (95% CI: 12.1–17.9%) with a significant standardized mean difference of −3.57 (95% CI: −4.64 to −2.44%, I2 = 94%) (Figure 5).
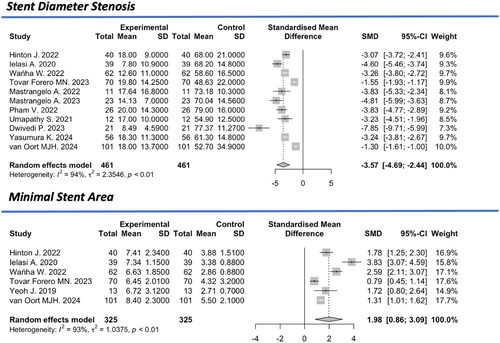
3.4 Long-Term Outcomes
Regarding long-term outcomes, over a mean follow-up period of 7.1 ± 7.8 months, the pooled incidence of all-cause mortality from 10 studies involving 447 patients was 4% (95% CI: 2%–6%, I2 = 0%) (Figure 1). During the same period, the incidence of myocardial infarction was 2% (95% CI: 0%–3%, I2 = 0%) (Appendix S1). The pooled rate of TLR during long-term follow-up was 6% (95% CI: 3%–10%, I² = 48%) (Figure 3). Given the relatively small sample sizes, a sensitivity analysis was conducted excluding studies with ≤ 10 patients to account for small-study effects. No significant differences were observed in hospital/30-day efficacy and safety outcomes, nor during the follow-up period.
4 Discussion
This study represents the first comprehensive systematic review and meta-analysis examining the use of IVL in treating lesions in underexpanded stents, incorporating angiographic (QCA) and intracoronary imaging (OCT, IVUS) data. Our findings indicate that IVL is a highly safe and effective strategy for underexpanded stent treatment, demonstrating a high overall procedural success rate and low peri-procedural complications. Immediate improvements were observed in SDS, MSA, as well as increases in MLD and MLA following IVL application. Additionally, the need for in-hospital/30-day TLR was rare with a notably low prevalence of all-cause mortality observed within the first 30 days (1%) and in the long-term (4%).
Stent under-expansion is a well-established independent risk factor for MACE after PCI due to stent thrombosis or ISR [29, 30]. The corresponding guidelines and expert consensus statements advocate for adequate lesion preparation with semi-compliant and noncompliant balloons, scoring balloons, or atherectomy [31, 32]. The gold standard option is the use of high-pressure balloon inflations to create a fracture of the underlying calcified plaque to correct the underexpanded stent. In-stent chronic total occlusions as well as lesions that are not responding to high pressure balloon constitutes a challenge for the operator with atherectomy being the next option. The application of rotational atherectomy (RA; Boston Scientific, Marlborough, MA) has been reported a well-tolerated modality for the treatment of ISR. However, in a real-world setting several concerns have been raised mainly for the potential procedural success and its limitation provoking by burr size, especially in vessels that are larger than 2 mm and by wire bias in angulated lesions [33, 34]. RA use has been also associated with complications mainly driven by no-reflow phenomena, strut embolization, and burr entrapment. Orbital atherectomy (OA; Cardiovascular Systems Inc., St. Paul, MN) is currently used for the modification of de novo calcified lesions to facilitate optimal stent deployment. The main proposed mechanisms of OA action is the direct ablation of calcium, stent ablation, mechanical modification of the calcium outside the stent -the so called “jackhammering” phenomenon- and thermal injury [35]. Its use may be preferred rather than rotational atherectomy for the treatment of underexpanded stents mainly in larger vessels and angulated lesions. The not so widely available excimer laser coronary atherectomy (ELCA) device is one of the most studied modalities for the treatment of underexpanded stents with undilatable lesions. It generates a photo-mechanical effect that modifies the underlying plaque, and when combined with high-pressure angioplasty, it corrects the malpositioned stent [33-37]. Nevertheless, the ablative effect of ELCA is minimal in cases of heavily calcified lesions, and it is not recommended for use in underexpanded stents of this nature.
Despite the limited data available on the use of IVL in underexpanded stents, and its current “off-label” status, it appears to be an alternative for interventional cardiologists, gaining ground compared to other techniques [30, 38]. IVL appears to be a safer option compared to RA, OA, and ELCA, primarily due to its autraumatic effect on the coronary wall and the relatively low learning curve associated with its use, making it a preferred modality even for operators with less experience in atherectomy techniques. IVL is also effective in underexpanded stent cases with the presence of neointimal hyperplasia or multiple stent layers [11]. Multiple stent layers as well as ostial position of the target lesion have been associated with higher expansion failure [9]. One of the concerns that may require further clarification is the assumption that the use of IVL as a bailout therapy after stent malposition may alter stent structure and affect drug delivery/polymer. Within this context, a bench study conducted in 2022 analyzed the stent structure using electron microscopy after subjecting it to eight shocks of IVL [39]. The study revealed that IVL does indeed impact the fluopolymer of DES, as evidenced by the observation of small tears, microcracks, and detachments of polymer flakes. However, these changes did not potentially reduce the antiproliferative effect and the overall integrity of the DES. Moreover, the researchers noted that similar features are observed after high-pressure balloon dilation of underexpanded stents, underscoring the minimal impact of IVL use on DES structure. Though, a potential concern for future investigation is the extent to which the stent's metallic scaffold absorbs the sonic energy of IVL, thereby limiting its effect on the calcium underneath.
This systematic review and meta-analysis is the largest summarizing the existing data adding in the literature, as it specifically shows that the coronary IVL systems are safe and effective in under-expanded stent treatment. Finally, our study demonstrated significant results not only in the first 30 days after the procedure but also in the long term (approximately 1 year), where the findings until now remained debatable. Further studies, however, may be necessary so that this treatment modality could be endorsed in future guidelines.
4.1 Limitations
First, the location and the nature of the lesions, therapy and duration of treatment among the studies were heterogeneous as well as follow-up of the studies. Second, in this meta-analysis, patients from different countries and health systems with different socioeconomic and ethnical background are pooled together, while the experience of operators was not taken into account. Third, some of the included studies reported outcomes for a small number of patients which may further increase the heterogeneity of the study, while intracoronary imaging data were limited. There were no studies to compare IVL with the other techniques and to test its long-term effectiveness.
5 Conclusion
Based on the results of the present meta-analysis, IVL is a highly safe and effective strategy for treating under-expanded stents, demonstrating a high overall procedural success rate and low peri-procedural complication rates. This efficacy is demonstrated by low MACE rates both short- and long-term, as well as favorable postprocedural intravascular imaging results. Immediate improvements were observed in SDS, MSA, as well as increases in MLD and MLA following IVL application. Future prospective cohorts will be required to validate our findings and expand our knowledge of the potential impact of IVL use in stent structure and drug delivery.
Acknowledgments
The authors have nothing to report.
Disclosure
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, M.S.




