Complications Leading to Death in Patients Supported by the Impella 5.5: Analysis From the FDA MAUDE Database
ABSTRACT
Background
The Impella 5.5 is increasingly being used in patients with cardiogenic shock, but experience with the device remains limited, and severe complications, including death, have been reported. This study aims to investigate causes of Impella 5.5-related deaths.
Methods
Data were extracted from the FDA Manufacturer and User Facility Device Experience (MAUDE) database using the search term “Impella 5.5” with the outcome “death.” Case details were reviewed and summarized.
Results
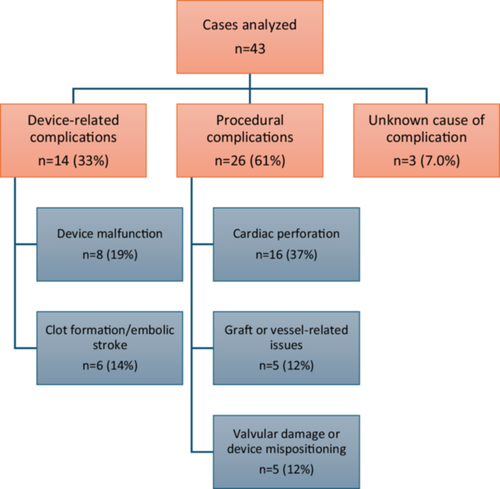
A total of 47 cases were reviewed. Of these, four cases were excluded as unrelated to the device. Among the 43 cases included, 40 involved either procedural-related or device-related complications. The remaining three cases had an indeterminant cause. Procedural complications were observed in 26 cases (61%). Of these, 16 cases (37%) involved cardiac perforation, which was frequently caused by improper placement or re-positioning of the device. Five cases (12%) involved graft/vessel-related complications, including bleeding, detachment from the aorta, or dissection, which were linked to technical difficulties during insertion or suturing. The remaining five cases (12%) involved valve damage or device malposition. Device-related complications were reported in 14 cases (33%). Of these, eight cases (19%) were device malfunctions including pump stoppage due to issues such as electrical failure, biomaterial ingestion, or lumen damage. Six patients (14%) experienced device-related clot formation and/or stroke.
Conclusions
Cardiac perforation emerged as the most frequent complication associated with Impella 5.5-related deaths, followed by device malfunction. These findings highlight the need for improved operational techniques, better device reliability, and refined safety protocols for Impella 5.5 usage.
1 Introduction
The Impella 5.5 is a temporary mechanical circulatory support device designed for use up to 14 days, and is typically used in patients with cardiogenic shock, severe heart failure, or end-stage heart failure awaiting definitive treatments such as heart transplantation or left ventricular assist device (LVAD) implantation [1]. Since its introduction to the US in October 2019, clinical use of the Impella 5.5 has increased [2]. Severe complications associated with the Impella 5.5, including death, have been reported in previous studies [3, 4]. However, the specific nature of these complications and the underlying factors contributing to fatal outcomes remain insufficiently explored.
2 Aims
This study aims to analyze fatal complications in patients supported by the Impella 5.5, using data from the FDA's Manufacturer and User Facility Device Experience (MAUDE) database, to identify key factors involved and provide insights for improving patient outcomes and optimizing device management.
3 Methods
3.1 Data Source and Extraction
This retrospective study utilized data from the FDA MAUDE database, a publicly accessible platform cataloging reports on medical device-related complications and outcomes. We searched the database using the term “Impella 5.5” and filtered results for cases with the outcome “death”.
3.2 Case Screening and Categorization
A total of 47 cases were identified. Subsequently, four were excluded as unrelated to the device. The remaining 43 cases were evaluated and categorized based on their association with the Impella 5.5. Of these, 40 cases involved complications that fell into two main categories: procedural-related complications and device-related complications. The remaining three cases had an indeterminant cause. Case details were then reviewed and summarized.
4 Results
Among the 43 cases analyzed, patient ages ranged from 20 to 79 years, with a mean of 63 years. Gender was reported in 28 cases, of whom 22 (79%) were male and six (21%) were female. The most common indication for Impella 5.5 support was hemodynamic instability following cardiac surgery (17 cases, 40%). Other indications included cardiogenic shock of unknown origin (14 cases, 33%), cardiogenic shock after acute myocardial infarction (10 cases, 23%), and end-stage heart failure awaiting LVAD or heart transplantation (2 cases, 5%). The duration of device support ranged from 1 to 22 days, with a mean of 7.1 days (excluding one outlier case with a duration of 144 days).
4.1 Procedural-Related Complications
Procedural complications were observed in 26 cases (61%). Cardiac perforation, the most frequent complication, occurred in 16 cases (37%), often attributed to improper positioning or re-positioning of the device within the left ventricle. Graft or vessel-related issues were reported in five cases (12%) and included bleeding, graft detachment from the aorta, or vessel dissection. In five other cases (12%), valvular damage or device mispositioning was identified.
4.2 Device-Related Complications
Device-related complications were noted in 14 cases (33%). Among these, eight cases (19%) involved device malfunction, with pump stoppage due to issues such as electrical failure, biomaterial ingestion, or lumen damage. Clot formation and embolic stroke were reported in six cases (14%), despite appropriate anticoagulation management. In three cases (7.0%), the cause of death remained uncertain, though sepsis was a shared complication.
5 Discussion
This study identifies procedural-related complications as the leading cause of death in patients supported by the Impella 5.5, with cardiac perforation being the most frequent. Improper placement or re-positioning of the device within the left ventricle emerged as a critical factor. Other procedural challenges, such as bleeding, vessel dissection, and graft detachment, underscore the technical complexities involved in device placement, particularly in patients with challenging anatomies or fragile cardiac tissue.
One contributing factor may be limited training and inexperience on best techniques for placement, re-positioning, or removal of the Impella 5.5. As the device was recently introduced, many facilities are still in the process of developing procedural expertise [4]. Therefore, increased practical experience may be necessary to master its proper manipulation and minimize complications. Additionally, anatomical variability—such as differences in ventricular or vessel sizes and tissue fragility—can further complicate standardization of the procedure. For instance, one reported case described how vessel size changes created difficulties during device removal. Similarly, several cases attributed cardiac perforation to friable cardiac tissue, which ultimately led to patient deaths (Figure 1).
Similar issues were reported with the Impella 5.0, the predecessor to the Impella 5.5. Structural modifications, such as removal of the pigtail and using a more rigid cannula and a shorter motor system, were incorporated into the Impella 5.5 to improve maneuverability in patients with difficult anatomy [5]. However, despite these improvements, Debenham et al. found that the Impella 5.5 still had a higher incidence of left ventricular perforation compared to the Impella 5.0 (13.3% vs. 1.7%, p < 0.001) [6]. This suggests that more comprehensive training and further refinement in device handling and design are necessary to reduce procedural complications (Figure 2).

Device-related complications were the second most frequent cause of death in this study. These included malfunctions such as pump stoppage, electrical failures, catheter kinks, and lumen damage, all of which rendered the device nonfunctional and ultimately led to patient deaths. Although less frequent, thrombus formation within the device was also reported, resulting in embolic strokes or device stoppage despite the use of anticoagulation. Debenham et al. also found that the Impella 5.5 had a higher incidence of pump stoppage compared to its predecessor [6], underscoring the need to improve the device's reliability and strategies for effective anticoagulation.
Our findings emphasize the importance of troubleshooting device-related complications to prevent malfunctions and reduce fatal outcomes. A notable observation from this study is that the Impella 5.5 was often discarded rather than returned to the manufacturer for analysis, limiting opportunities to identify and address design flaws. Encouraging clinicians to return malfunctioning devices for investigation could provide valuable insights for future design improvements and device management strategies and ultimately enhance patient safety.
5.1 Limitations
There were several limitations in this study. First, the sample size (n = 43) was small, limiting the generalizability of our conclusions. Second, data was obtained retrospectively and was limited in clinical detail to allow for a comprehensive analysis of the contributors to fatal outcomes, clinical/procedural context, and patient characteristics/comorbidities. Third, the lack of a control group prevented a comparative analysis of patient outcomes (e.g., Impella 5.0 vs. 5.5 outcomes). Lastly, underreporting bias is likely given the nature of the MAUDE database relying on voluntary submissions which may not reflect all adverse outcomes.
6 Conclusions
Cardiac perforation was the most frequent complication associated with Impella 5.5-related mortality, followed by device malfunction. These findings underscore the need for improved procedural techniques, enhanced clinical management, and the development of standardized safety protocols for device usage. Additionally, they highlight the importance of regular troubleshooting of device malfunctions and improving the reliability of the Impella 5.5 to mitigate complications and optimize patient outcomes in the future.
Acknowledgments
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




