Dual Antiplatelet Therapy Prior to Percutaneous Coronary Intervention for Acute Coronary Syndrome: Prevalence and Outcomes in Contemporary Practice
ABSTRACT
Background
Dual antiplatelet therapy (DAPT) is standard following percutaneous coronary intervention (PCI) for acute coronary syndrome (ACS). Preloading is the practice of administering both aspirin and a P2Y12 inhibitor before PCI. DAPT preloading is common practice, however clinical trial evidence demonstrating benefit is lacking.
Aims
This study aimed to examine the prevalence and associated clinical outcomes of DAPT before PCI for ACS in a contemporary population of Australian patients.
Methods
Data on consecutive PCI procedures from patients included in the Victorian Cardiac Outcomes Registry (VCOR) from 2014 to 2021 was collected and stratified by administration of DAPT before PCI versus single, or no, antiplatelet therapy.
Results
In total, 42,453 consecutive PCI procedures for ACS were included. Of these, 33,520 (79%) patients were either preloaded or already on DAPT before PCI. Patients on DAPT were younger (63.9 vs. 65.1, p < 0.001) and generally had fewer comorbidities. Unadjusted outcomes were more favorable with pre-loading with lower in-hospital mortality with DAPT (2.6% vs. 5.6%, p < 0.001), and 30-day cardiovascular mortality (0.3% vs. 0.4%, p = 0.039). 30-day major adverse cardiovascular events (MACE) (5.5% vs. 8.8%, p < 0.001) was similarly lower in the preloaded group. Major bleeding in hospital was less common in patients on DAPT (1.0% vs. 1.7%, p < 0.001). However, following adjustment for covariates, there was no difference in in-hospital or 30-day all-cause mortality, MACE or stent thrombosis between groups.
Conclusions
DAPT before PCI is common in ACS but not independently associated with improvements in in-hospital mortality, MACE, or stent thrombosis.
1 Introduction
Patients with acute coronary syndrome (ACS), and especially ST-elevation myocardial infarction (STEMI), require urgent revascularisation with percutaneous coronary intervention (PCI). Dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor is standard therapy following PCI for ACS to prevent stent thrombosis and recurrent myocardial infarction [1, 2]. Given PCI often follows on from diagnostic coronary angiography for ACS, preloading with a P2Y12 inhibitor alongside aspirin is often undertaken, variably timed from immediately before the PCI up to 24 h preprocedure as part of a preloading strategy. The main theoretical benefit of early dual antiplatelet loading is achieving maximal antiplatelet effect at the time of stent deployment, thereby mitigating the risk of stent thrombosis, which carries an estimated 20% risk of in-hospital mortality [3]. Conversely, the main limitation of P2Y12 inhibitor preloading is an increased risk of bleeding—particularly coronary artery bypass graft (CABG) associated bleeding—if early surgical revascularisation is required following diagnostic angiography. As such, DAPT before determining coronary anatomy has been associated with delays of CABG in a subset of patients referred for angiography [4].
While P2Y12 inhibitor preloading is commonly practiced, data are mixed regarding the efficacy of this approach. The CREDO trial showed a time dependent clinical benefit of clopidogrel loading [5]. However, subsequent trials have shown no difference in preloading before PCI versus in-lab or post PCI antiplatelet therapy (in both ACS and non-ACS cohorts) [6-9]. As such, international practice guidelines have evolved over the last decade. In patients with ACS, American College of Cardiology (ACC) guidelines currently recommend a loading dose of DAPT before or at least at the time of PCI but do not recommend preloading before coronary anatomy is determined [10]. In contrast, the European Society of Cardiology (ESC) guidelines recommend preloading with DAPT for patients with STE-ACS as soon as the diagnosis is established but do not specify an exact time frame for doing so. ESC guidelines do not recommend preloading for NSTE-ACS patients in whom coronary anatomy is unknown, and early invasive management is planned (< 24 h) [11]. There are limited data regarding the prevalence of DAPT before PCI for ACS in Australian cohorts. This study aimed to examine the prevalence of DAPT preloading in a contemporary population of Australian patients and determine its influence on clinical outcomes.
2 Methods
The Victorian Cardiac Outcomes Registry (VCOR) is a state-wide, multicenter clinical quality registry in Victoria, Australia coordinated by Monash University. It was established in 2013 and is contributed to by all public and private PCI-capable centers in Victoria with a steering committee comprising of representatives from contributing centers. The VCOR methodology has previously been described [12]. Following institutional ethics approval, data from all adult patients who underwent PCI for an indication of STEMI or NSTEMI between 1 January 2014 and 31 December 2021 were included. Patients with stable angina, unstable angina, and patients who received PCI ≥ 7 days following the index ACS, were excluded.
Patients were analyzed in two groups based on the presence or absence of DAPT (aspirin and a P2Y12 inhibitor consisting of either clopidogrel, ticagrelor, or prasugrel) before PCI. We defined those that were on DAPT as “preloaded.” The non-preloaded group comprised of patients that received only single or no antiplatelet therapy before PCI. Baseline clinical characteristics, procedural characteristics, medication characteristics and clinical outcomes were analyzed by univariate analysis. Lesion complexity was defined as either A, B1, B2, or C according to the American College of Cardiology/American Heart Association (ACC/AHA) classification guidelines [13]. Data collected on clinical outcomes during admission and at 30 days included all-cause mortality, new renal impairment, shock, new MI, subsequent PCI, target vessel revascularisation (TVR), target lesion revascularisation (TLR), CABG, stent thrombosis, stroke, major bleeding event, major adverse cardiovascular event (MACE), and major adverse cardiac and cerebrovascular event (MACCE). Utilizing Bleeding Academic Research Consortium (BARC) [14] definitions, major bleeding unrelated to CABG was defined as either BARC Type 3 (overt haemorrhage with haemoglobin drop of > 3 g/dL, intracranial bleeding, cardiac tamponade or requires transfusion) or BARC Type 5 (probable or definite fatal haemorrhage).
Continuous variables are expressed as means with standard deviations while categorical variables are shown as the number of patients in percentages. Comparison of categorical and continuous variables in addition to assessment of statistical significance were performed using χ2 or t-tests accordingly. Tests were two-tailed with a p < 0.05 considered to be significant.
Multivariate logistic regression was performed to estimate the odds ratio (OR) of relevant clinical outcomes including in-hospital and 30-day all-cause mortality, MACE, major bleeding, and stent thrombosis. Major confounders including age, gender, type of myocardial infarction (STEMI/NSTEMI), BMI, eGFR, diabetes mellitus, peripheral vascular disease (PVD), cerebrovascular disease (CeVD), previous CABG, previous PCI, chronic anticoagulant therapy, left ventricular ejection fraction (LVEF), cardiogenic shock, out of hospital cardiac arrest (OOHCA), in-hospital pre-procedure cardiac arrest, in-hospital pre-procedure intubation and DAPT on discharge were included and adjusted for in the multivariate model.
3 Results
Forty-two thousand four hundred and fifty-three consecutive PCI procedures were included and analyzed across the 8-year study period. Of these, 33,520 (79%) patients were on DAPT before PCI. Baseline clinical characteristics are described in Table 1. Patients who were preloaded were younger (63.9 vs. 65.1 years, p < 0.001) and were less likely to present with comorbidities like peripheral vascular disease (2.8% vs. 4.4%, p < 0.001), cerebrovascular disease (3.1% vs. 5.1%, p < 0.001), eGFR ≤ 60 (19.9% vs. 23.9%, p < 0.001) and prior CABG (4.4% vs. 5.6%, p < 0.001). In contrast, male gender (75.8% vs. 76.1%, p = 0.572), a history of diabetes mellitus (21.0% vs. 21.4%, p = 0.461), STEMI indication for PCI (47% vs 46.2%, p = 0.166) and a history of previous PCI (17.2% vs. 17.9%, p = 0.160) were similar between the two groups.
| Preloading (n = 33,520) | No preloading (n = 8933) | p value | ||
|---|---|---|---|---|
| Age | 63.9 ± 12.7 | 65.12 ± 12.7 | < 0.001 | |
| BMI (kg/m2) | 28.8 ± 5.6 | 28.6 ± 5.6 | < 0.001 | |
| eGFR mL/min/1.73 m2 | 94.2 ± 41.0 | 88.9 ± 39.8 | < 0.001 | |
| Male | Yes | 25416 (75.8%) | 6799 (76.1%) | 0.572 |
| No | 8104 (24.2%) | 2134 (23.9%) | ||
| Diabetes mellitus | 7053 (21.0%) | 1911 (21.4%) | 0.461 | |
| Peripheral vascular disease | 934 (2.8%) | 395 (4.4%) | < 0.001 | |
| Cerebrovascular disease | 1030 (3.1%) | 454 (5.1%) | < 0.001 | |
| Previous CABG | 1486 (4.4%) | 499 (5.6%) | < 0.001 | |
| Previous PCI | 5782 (17.2%) | 1597 (17.9%) | 0.160 | |
| Chronic oral anticoagulant therapy | 1619 (4.8%) | 592 (6.6%) | < 0.001 | |
| LVEF | Mild to normal (> 44%) | 24184 (78.6%) | 6395 (77.1%) | < 0.001 |
| Moderate (35%–44%) | 4560 (14.8%) | 1244 (15.0%) | ||
| Severe (< 35%) | 2032 (6.6%) | 660 (8.0%) | ||
| eGFR | > 60 | 24846 (80.1%) | 6221 (76.1%) | < 0.001 |
| ≤ 60 | 6162 (19.9%) | 1954 (23.9%) | ||
| Indication for PCI | STEMI | 15766 (47.0%) | 4128 (46.2%) | 0.166 |
| NSTEMI | 17754 (53.0%) | 4805 (53.8%) | ||
| Cardiogenic shock | 1331 (4.0%) | 632 (7.1%) | < 0.001 | |
| Out-of-hospital cardiac arrest | 1168 (3.5%) | 587 (6.6%) | < 0.001 | |
| In-hospital pre procedure cardiac arrest | 852 (2.5%) | 308 (3.4%) | < 0.001 | |
| In-hospital pre procedure intubation | 939 (2.8%) | 488 (5.5%) | < 0.001 | |
Patients were less likely to be preloaded if they presented for their index PCI with cardiogenic shock (4.0% vs. 7.1%, p < 0.001) or an OOHCA (3.5% vs. 6.6%, p < 0.001), in-hospital preprocedure cardiac arrest (2.5% vs. 3.4%, p < 0.001) or received in-hospital preprocedure intubation (2.8% vs. 5.5%, p < 0.001). Procedurally, radial access was more common in patients that were preloaded with DAPT (69.9% vs. 57.1%, p < 0.001) (Table 2).
| Preloading (n = 33,520) | No preloading (n = 8933) | p value | |||
|---|---|---|---|---|---|
| Procedural characteristics | PCI access | Brachial/radial | 23444 (69.9%) | 5104 (57.1%) | < 0.001 |
| Femoral | 10076 (30.1%) | 3829 (42.9%) | |||
| Lesion location | RCA | 11677 (34.8%) | 3179 (35.6%) | < 0.001 | |
| LAD | 13641 (40.7%) | 3584 (40.1%) | |||
| Cx | 7286 (21.7%) | 1833 (20.5%) | |||
| Left main | 462 (1.4%) | 177 (2.0%) | |||
| Graft | 454 (1.4%) | 160 (1.8%) | |||
| Lesion type (complexity) | A or B1 | 13173 (39.3%) | 3072 (34.4%) | < 0.001 | |
| B2 or C | 20347 (60.7%) | 5861 (65.6%) | |||
| Total stent number | No stent | 1806 (5.4%) | 773 (8.7%) | < 0.001 | |
| 1 stent | 22736 (67.8%) | 6088 (68.2%) | |||
| 2 stents | 6881 (20.5%) | 1643 (18.4%) | |||
| 3+ stents | 2097 (6.3%) | 429 (4.8%) | |||
| Type of stent | Any DES | 29340 (87.5%) | 7311 (81.8%) | < 0.001 | |
| Any BVS no DES | 38 (0.1%) | 14 (0.2%) | |||
| BMS only | 2326 (6.9%) | 834 (9.3%) | |||
| other stent | 10 (< 0.1%) | 1 (< 0.1%) | |||
| POBA | 1241 (3.7%) | 480 (5.4%) | |||
| No stent/balloon | 565 (1.7%) | 293 (3.3%) | |||
| Total stent number | 1.3 ± 0.7 | 1.2 ± 0.7 | < 0.001 | ||
| Total stent length (mm) | 28.3 ± 16.4 | 25.1 ± 15.5 | < 0.001 | ||
Ticagrelor was more commonly used than the thienopyridine P2Y12 inhibitors, clopidogrel and prasugrel (59.5% vs. 23.4%). However, prasugrel's withdrawal from the Australian market in July 2020 occurred during the study period. Patients were less likely to be preloaded with DAPT when also administered glycoprotein IIb/IIIa inhibitors (13.7% vs. 23.0%, p < 0.001), and were less likely to be discharged on oral anticoagulation therapies if they had been preloaded (7.6% vs. 11.4%, p < 0.001). Preloaded patients were also more likely to be discharged on DAPT (96.4% vs. 92.9%, p < 0.001) (Table 3).
| Preloading (n = 33,520) | No preloading (n = 8933) | p value | ||
|---|---|---|---|---|
| 24 h before and/or during PCI | No antiplatelet | 0 (0%) | 1057 (11.8%) | < 0.001 |
| Aspirin | 33520 (100.0%) | 6526 (74.2%) | < 0.001 | |
| Thienopyridine | 9277 (27.7%) | 653 (7.3%) | < 0.001 | |
| Ticagrelor | 24527 (73.2%) | 727 (8.1%) | < 0.001 | |
| Glycoprotein IIb/IIIa inhibitor | 4593 (13.7%) | 2056 (23.0%) | < 0.001 | |
| On discharge from hospital | Aspirin | 31870 (97.6%) | 8072 (95.7%) | < 0.001 |
| Thienopyridine | 9425 (28.9%) | 2930 (34.8%) | < 0.001 | |
| Ticagrelor | 22785 (69.8%) | 5234 (62.1%) | < 0.001 | |
| DAPT | 31469 (96.4%) | 7831 (92.9%) | < 0.001 | |
| Beta blockers | 25990 (79.6%) | 6924 (82.1%) | < 0.001 | |
| ACEi/ARB | 25526 (78.2%) | 6587 (78.1%) | < 0.001 | |
| Statin | 31070 (95.2%) | 8018 (95.1%) | 0.199 | |
| Other lipid lowering therapies | 2275 (7.0%) | 650 (7.7%) | 0.008 | |
| Oral anticoagulation therapies | 2042 (7.6%) | 894 (11.4%) | < 0.001 | |
Unadjusted in-hospital mortality (2.6% vs. 5.6%, p < 0.001) and 30-day mortality (3.3% vs 6.4%, p < 0.001) were less frequent in patients that were preloaded with DAPT. 30-day cardiovascular mortality was also lower in the DAPT group (0.3% vs. 0.4%, p = 0.039). The DAPT preloading group had lower incidence of in-hospital new MI (0.7% vs. 1.0%, p = 0.002) and 30-day new MI (1.3% vs. 1.6%, p = 0.019). The rates of in-hospital new stroke (0.4% vs. 0.6%, p = 0.05) was also lower with preloading. 30-day MACE (5.5% vs. 8.8%, p < 0.001) and 30-day MACCE (5.9% vs. 9.4%, p < 0.001) were lower in patients that were preloaded. Patients that were preloaded experienced lower rates of both major bleeding in-hospital (1.0% vs. 1.7%, p < 0.001) and major bleeding at 30-days (1.4% vs. 2.2%, p < 0.001). Preloading was also associated with a shorter median length of stay (3 vs. 4 days, p < 0.001). Rate of rehospitalisation (12.8% vs. 14.3%, p < 0.001) and rate of unplanned cardiac rehospitalisation (8.3% vs. 9.7%, p < 0.001) were also both lower in preloaded patients (Table 4). However, preloading made no difference to both the rate of in-hospital stent thrombosis (0.4% vs. 0.5%, p = 0.220) and 30-day stent thrombosis (0.7% vs. 0.8%, p = 0.194).
| Preloading (n = 33,520) | No preloading (n = 8933) | p value | ||
|---|---|---|---|---|
| In-hospital | Mortality | 883 (2.6%) | 502 (5.6%) | < 0.001 |
| New renal impairment | 1610 (5.4%) | 449 (5.7%) | 0.426 | |
| Shock | 984 (2.9%) | 511 (5.7%) | < 0.001 | |
| New/recurrent MI | 232 (0.7%) | 91 (1.0%) | 0.002 | |
| PCI | 2184 (6.5%) | 410 (4.6%) | < 0.001 | |
| TVR (PCI) | 382 (1.1%) | 93 (1.0%) | 0.431 | |
| TLR | 280 (0.8%) | 74 (0.8%) | 0.949 | |
| CABG | 334 (1.0%) | 133 (1.5%) | < 0.001 | |
| Stent thrombosis | 147 (0.4%) | 48 (0.5%) | 0.220 | |
| Major bleed | 344 (1.0%) | 155 (1.7%) | < 0.001 | |
| Stroke | 136 (0.4%) | 50 (0.6%) | 0.05 | |
| At 30 days | Mortality | 1108 (3.3%) | 575 (6.4%) | < 0.001 |
| Cardiovascular Mortality | 86 (0.3%) | 35 (0.4%) | 0.039 | |
| New MI | 442 (1.3%) | 147 (1.6%) | 0.019 | |
| New stent thrombosis | 220 (0.7%) | 70 (0.8%) | 0.194 | |
| Major bleed | 468 (1.4%) | 199 (2.2%) | < 0.001 | |
| New stroke | 190 (0.6%) | 66 (0.7%) | 0.062 | |
| TVR | 601 (1.8%) | 149 (1.7%) | 0.426 | |
| TLR | 423 (1.3%) | 114 (1.3%) | 0.915 | |
| CABG | 468 (1.4%) | 184 (2.1%) | < 0.001 | |
| Major adverse cardiovascular event | 1832 (5.5%) | 786 (8.8%) | < 0.001 | |
| Major adverse cardiac and cerebrovascular event | 1974 (5.9%) | 838 (9.4%) | < 0.001 | |
| Length of stay (days) | 3 | 4 | < 0.001 | |
| Rehospitalisation | 4182 (12.8%) | 1202 (14.3%) | < 0.001 | |
| Unplanned Cardiac Rehospitalisation | 2776 (8.3%) | 863 (9.7%) | < 0.001 | |
Following adjustment for covariates, there was no difference in in-hospital and 30-day mortality (OR 1.10; 95% confidence interval [CI]: 0.86–1.42, p = 0.45 and OR 1.09; 95% CI: 0.90–1.31, p = 0.39 respectively). There was also no difference in 30-day MACE (OR 1.01, 95% CI: 0.89–1.15, p = 0.82) and no independent association with a lower risk of in-hospital stent thrombosis (OR 1.11, 95% CI: 0.75–1.64, p = 0.60) or 30-day stent thrombosis (OR 1.05, 95% CI: 0.77–1.43, p = 0.77) (Figure 1). However, preloading was found to be independently associated with a lower risk of in-hospital major bleeding (OR 0.82, 95% CI: 0.66–1.02, p = 0.08) and 30-day major bleeding (OR 0.79, 95% CI: 0.65–0.96, p = 0.02). Subgroup analysis revealed that a lower risk of 30-day MACE was more commonly associated with thienopyridine use as compared to ticagrelor (OR: 0.81, 95% CI: 0.70–0.94, p = 0.006) (Figure 2).
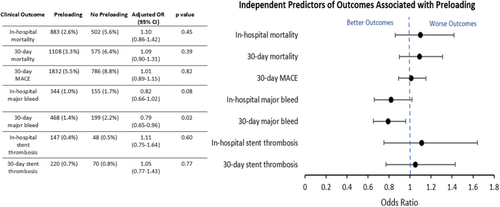
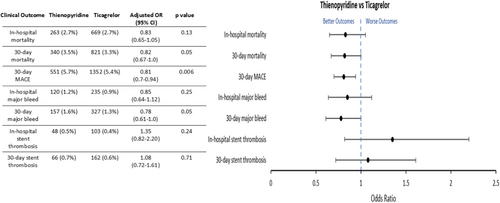
Given varying guideline recommendations in STE-ACS and NSTE-ACS, subgroup analysis comparing the impact of DAPT on clinical outcomes was performed in the STEMI and NSTEMI cohorts (Table 5). There was no statistically significant difference with preloading in either group on in-hospital or 30-day mortality (Table 5). The only statistically significant finding was the association of DAPT preloading with a reduction in 30-day major bleeding in NSTEMI patients (adjusted OR: 0.70; 95% CI: 0.52–0.93, p = 0.01) (Table 5B).
| A: Clinical outcomes associated with STEMI | ||||||
|---|---|---|---|---|---|---|
| Preloading (n = 15,766) | No preloading (n = 4128) | p value | Adjusted OR (95% CI) | Adjusted p value | ||
| STEMI | In hospital mortality | 740 (4.7%) | 428 (10.4%) | < 0.001 | 1.27 (0.92–1.73) | 0.14 |
| 30-day mortality | 879 (5.6%) | 472 (11.4%) | < 0.001 | 1.14 (0.89–1.46) | 0.29 | |
| In hospital major bleed | 236 (1.5%) | 106 (2.6%) | < 0.001 | 0.88 (0.67–1.16) | 0.37 | |
| 30-day major bleed | 298 (1.9%) | 123 (3.0%) | < 0.001 | 0.88 (0.68–1.13) | 0.32 | |
| In-hospital stent thrombosis | 127 (0.8%) | 42 (1.0%) | 0.19 | 1.06 (0.69–1.63) | 0.78 | |
| 30-day stent thrombosis | 161 (1.0%) | 51 (1.2%) | 0.23 | 1.01 (0.69–1.48) | 0.95 | |
| 30-day MACE | 1255 (8.0%) | 582 (14.1%) | < 0.001 | 0.99 (0.83–1.18) | 0.95 | |
| B: Clinical outcomes associated with NSTEMI | ||||||
|---|---|---|---|---|---|---|
| Preloading (n = 17,754) | No preloading (n = 4805) | p value | Adjusted OR (95% CI) | Adjusted p value | ||
| NSTEMI | In hospital mortality | 143 (0.8%) | 74 (1.5%) | < 0.001 | 0.89 (0.58–1.36) | 0.58 |
| 30-day mortality | 229 (1.3%) | 103 (2.1%) | < 0.001 | 1.01 (0.74–1.38) | 0.95 | |
| In hospital major bleed | 108 (06%) | 49 (1.0%) | 0.002 | 0.74 (0.51–1.05) | 0.09 | |
| 30-day major bleed | 170 (1.0%) | 76 (1.6%) | < 0.001 | 0.70 (0.52–0.93) | 0.01 | |
| In-hospital stent thrombosis | 20 (0.1%) | 19 (0.4%) | 0.82 | 1.41 (0.51–3.91) | 0.51 | |
| 30-day stent thrombosis | 59 (0.3%) | 19 (0.4%) | 0.51 | 1.12 (0.63–1.97) | 0.70 | |
| 30-day MACE | 577 (3.2%) | 204 (4.2%) | < 0.001 | 1.04 (0.86–1.26) | 0.71 | |
4 Discussion
This study demonstrated that preloading with DAPT is common, occurring in 79% of a contemporary Australian ACS cohort undergoing PCI. The indication for PCI (STEMI vs NSTEMI) was similar between groups. Preloaded patients were found to be younger, had fewer comorbidities and less frequently presented with high-risk presentations such as cardiogenic shock and OOHCA. Preloaded patients had a shorter median length of stay, lower rates of 30-day rehospitalisation and were also less likely to be rehospitalised for unplanned cardiovascular causes. Following adjustment for covariates, there was no difference between the two groups in all-cause mortality, MACE and stent thrombosis in-hospital and at 30-days.
Preloading with DAPT is believed to maximize platelet inhibition, minimize thrombotic complications related to PCI and avoid the use of more potent agents like glycoprotein IIb/IIIa inhibitors. DAPT preloading has been studied in multiple randomized clinical trials (RCTs) over the last two decades. However, there has been no epidemiological data to date on how widely practiced DAPT preloading is in clinical practice in the contemporary Australian population. The Australian Clinical Guidelines for the Management of Acute Coronary Syndromes, published in 2016, does not recommend preloading with a P2Y12 inhibitor due to limited data in the literature supporting its use, but does recommend preloading with prasugrel immediately upon diagnosis in patients receiving primary PCI for STEMI [15]. The ACC guidelines make no distinction between preloading and periprocedural DAPT administration (non-preloaded) and therefore do not recommend preloading before coronary anatomy is determined [10]. Recently updated ESC guidelines outline a preference for preloading in STE-ACS, but not in NSTE-ACS if early invasive management is planned and coronary anatomy is unknown [11, 16]. Despite a lack of cohesive evidence in the literature, the present study found that preloading was common at 79% in an Australian ACS cohort undergoing PCI. A comparative Swedish registry of 69,211 patients from 2000 to 2018 reported a prevalence of preloading in patients with NSTE-ACS to be 92.4% [17]. Another study from the same registry reported a high prevalence of more than four out of five patients with STEMI being preloaded from 2005 to 2016 in a cohort of 44,804 patients [18]. Similar analysis in an Austrian cohort comprising of 5955 patients who received PCI for STEMI from 2005 to 2009 reported a high prevalence of preloading at 82% [19]. The high prevalence of the practice of preloading reflected by the results of this study as well as that of the Swedish and Austrian registry studies could be attributed to the recommendation before 2018 being adopted and implemented by hospitals in these countries.
We identified that the preloaded group was less likely to require glycoprotein IIb/IIIa inhibitors, similar to previous studies among STE-ACS patients [18, 19]. These agents are typically used as bailout therapy in complex PCI with angiographic evidence of slow or no-reflow or large intraluminal thrombus burden [11]. Our and others' finding of lower glycoprotein IIb/IIIa inhibitor use in preloaded patients suggests that preprocedural DAPT has a beneficial effect on reducing the incidence and/or harmful effects of these angiographic findings. We also observed that preloading was less frequent in those with high-risk presentations like cardiogenic shock, OOHCA, in-hospital preprocedure cardiac arrest or require in-hospital preprocedure intubation, conditions previously demonstrated to be associated with adverse outcomes [20, 21].
This study showed that preloading was independently associated with a lower risk of major bleeding both in-hospital and at 30 days. The early randomized controlled studies, CURE and CLARITY, reported no increase in major bleeding associated with preloading with clopidogrel [6, 22]. Registry data have also demonstrated that DAPT is not associated with an increased bleeding risk [18, 19]. In contrast, the ACCOAST trial and a Swedish registry study reported an increased rate of TIMI major bleeding at 7 days (HR 1.90, 95% CI: 1.19–3.02, p-0.006) [7] and increased risk of in-hospital bleeding associated with preloading (OR: 1.49, 95% CI: 1.06–2.12, p = 0.02) [17]. Furthermore, a meta-analysis of 7 RCTs comprising 13,226 patients with NSTE-ACS found preloading was associated with an increased risk of major bleeding (OR: 1.51, 95% CI: 1.16–1.97, I2 = 41%) [23]. The relationship we observed between preloading and major bleeding is unexpected and contrasts to previous studies. One possible explanation is that patients at high risk of major bleeding at index presentation may not have been preloaded with DAPT, whereas those with no clear contraindications were more readily preloaded. Another explanation may be that our study had lower risk patients for bleeding as compared to previous data with the observed bleeding relationship due to variance in this setting. Antiplatelet timing itself was unable to be specifically assessed in this registry study but may also contribute to risk.
We found that preloading was not independently associated with a lower risk of mortality, 30-day MACE and stent thrombosis which was consistent in subgroup analysis of patients presenting with STE-ACS and NSTE-ACS. This is consistent with registry data in NSTE-ACS [17] and STE-ACS [18] cohorts and meta-analysis of 7 RCTs in NSTE-ACS which found no improvement in mortality or stent thrombosis risk at 30 days [23]. There have been multiple RCTs examining the effects of DAPT preloading on clinical outcomes in ACS cohorts. Studies examined the effects of preloading have generally randomized a single P2Y12 inhibitor—clopidogrel, prasugrel, or ticagrelor (and not compared between them). Studies like CREDO, CURE, CLARITY and the Austrian registry that examined the effects of preloading with clopidogrel have generally demonstrated benefits with antiplatelet preloading [5, 6, 19, 22]. In contrast, studies including ACCOAST, ATLANTIC, and DUBIUS that examined the effects of preloading with other single P2Y12 inhibitors have failed to determine benefit [7, 8, 24]. Unlike clinical trials, this study utilized real world clinical data examining all P2Y12 inhibitors at operator discretion. We conducted subgroup analysis examining ticagrelor versus thienopyridine which suggested that thienopyridine use was more likely to be associated with a lower risk of 30-day MACE (OR: 0.81, 95% CI: 0.7–0.94, p = 0.006). This, in addition to trial data, suggests that clinical outcomes associated with DAPT preloading may be dependent on the specific P2Y12 inhibitor used.
The lack of consensus with regard to the mortality benefits and bleeding risk associated with preloading could be due to population differences between the various registries in addition to data collection. The Swedish cohort studies had sample sizes that were comparable and larger, comprising of 44,804 and 69,211 patients. They also only included patients who received PCI for either STE-ACS or NSTE-ACS. In contrast, the Austrian study only had a sample size of 5955 patients and only included patients receiving PCI for STEMI. Unlike both studies, this study included a large population presenting with both STE-ACS and NSTE-ACS.
As above, this study provides a contemporary analysis of the clinical application and relevance of pretreatment with DAPT in a large population of patients. However, the findings of this study should be interpreted in the context of its limitations. “Preloading” by traditional definition was difficult to establish for those that were already on DAPT for alternative indications, and as such, the time point of preloading, and the prevalence of those who were already established on DAPT before presenting with ACS, was not identifiable. Therefore, the lack of clearly defined time frame for the preloaded group might confound the findings of the study by conferring an unfair advantage to those on chronic DAPT due to a pre-existing baseline level of platelet inhibition. Despite its strength as a large cohort study, this study is also limited by its retrospective, observational design and subsequent vulnerability to bias that occur in all large-scale registries. As an analysis of observational data, unmeasured confounders may exist despite adjustment for covariates. Although major confounders including age, gender, type of myocardial infarction (STEMI/NSTEMI), BMI, eGFR, diabetes mellitus, PVD, CeVD, previous CABG, previous PCI, chronic anticoagulant therapy, LVEF, cardiogenic shock, OOHCA, in-hospital pre-procedure cardiac arrest, in-hospital pre-procedure intubation and DAPT on discharge were adjusted for in multivariate logistic regression, variables known to influence clinical outcomes after PCI such as heart rate, occurrence of no-reflow/slow blood flow after PCI were not [25, 26]. Moreover, due to the absence of data on recurrent dynamic ST-T wave changes and refractory arrhythmia, it is likely that not all the high risk presentations would have been adjusted for in the multivariate analysis together with cardiogenic shock, in-hospital cardiac arrest and in-hospital intubation. Consequently, this may have led to worse clinical outcomes in the non-preloaded group as well as the lack of ischaemic benefit expected with preloading. Lastly, data on pharmacological characteristics may not be reflective of true medication adherence given the difficulty of verifying it in a study population this size.
In conclusion, DAPT before PCI in ACS is common in the Australian population. Yet, despite its widespread use, preloading was not found to be independently associated with improvements in in-hospital mortality, MACE, or stent thrombosis.
5 Acknowledgments
Open access publishing facilitated by The University of Melbourne, as part of the Wiley - The University of Melbourne agreement via the Council of Australian University Librarians.
Conflicts of Interest
The authors declare no conflicts of interest.




