Steerable Delivery Sheath for Optimized LAA Closure: First Experience and Procedural Outcomes
Matthias Mezger and Christina Paitazoglou are shared first authorship.
ABSTRACT
Background
The new Amplatzer Steerable Delivery Sheath is a delivery system designed to improve ease-of-use and procedural results of left atrial appendage closure (LAAC). We aimed to compare procedural results after switching our LAAC program at a tertiary care center with the Amulet device to the Steerable Delivery Sheath, with a control group of LAAC employing the standard sheath.
Methods
The first n = 32 consecutively treated patients at our site using the Amulet device with the Steerable Delivery Sheath were included in this retrospective analysis. As a control-group, n = 39 consecutive patients treated with the Amulet device before the switch to the new sheath were used.
Results
LAAC was successful in all patients in both groups (100%). Contrast use and fluoroscopy time were numerically higher in the steerable sheath group (steerable sheath vs. control group: contrast use 70 ± 23 vs. 55 ± 50 mL, p = 0.09, fluoroscopy time 12.7 ± 4.6 vs. 10.2 ± 6.6 min, p = 0.3). Fluoroscopy time and dose decreased after 3 months while contrast use remained unchanged. Complete sealing rate was high in both groups (steerable sheath vs. control group 97% vs. 95%, p > 0.9) and periprocedural complication rate was low, without any periprocedural stroke, vascular complications, or death in both cohorts.
Conclusions
LAAC with the Amplatzer Amulet steerable delivery sheath is feasible and safe. Fluoroscopy time and dose suggest a learning curve with the new sheath.
1 Introduction
Atrial fibrillation (AF) is the most frequent cardiac arrhythmia and the incidence will continue to increase over the next 30 years, especially in countries with middle socio-demographic index, becoming one of the largest epidemics and public health challenges [1]. Non-vitamin K oral anticoagulants (NOACs) have a favorable risk-benefit profile as compared to warfarin therapy, yet extracranial bleeding including gastrointestinal bleeding is still a clinical problem especially in elderly, comorbid patient cohorts [2]. Interventional left atrial appendage closure (LAAC) is able to reduce the stroke and bleeding risk in AF patients with relative or absolute contraindication for long-term oral anticoagulation (OAC) [3-7]. In the last decade, a wealth of data has emerged on the safety of LAAC accompanied with a rapidly growing adoption of the procedure in clinical practice. Currently, there are two devices predominantly used worldwide for LAAC: The Watchman FLX (Boston Scientific) and the Amplatzer Amulet (Abbott). Safety of both devices in high-risk AF-patients with increased bleeding risk has been demonstrated for the Watchman FLX in the EWOLUTION registry [8] and for the Amplatzer Amulet device in the Amulet Observational Study [9].
However, several issues in the field of LAAC remain including the limited randomized efficacy data, peri-device leak (PDL), device-related thrombus (DRT), and the ongoing refinement of procedural techniques. The emergence of various occluder devices with enhanced sealing mechanisms and the availability of steerable delivery sheaths may further enhance the operator's ability to attain complete closure of the LAA although studies supporting this assumption remain necessary.
Recently, a new Amplatzer Amulet steerable delivery sheath has been developed for optimal LAA occlusion to improve ease of use and procedural results of interventional LAAC. This next generation delivery system has potentially advantages regarding maneuverability, precision and control in both simple and challenging LAA anatomies. However, systematic data regarding the feasibility, safety and potential advantages are lacking. We therefore aimed to compare the procedural results between patients who received LAAC with the Amulet device via the standard delivery sheath and patients who received the Amulet device with the new steerable delivery sheath.
2 Methods
2.1 Study Design
We performed a single-center, retrospective analysis of the first n = 32 consecutively treated patients at our site (University Heart Center Lübeck, Germany) using the Amulet device with the steerable delivery sheath. As a control group, n = 39 consecutive patients treated with the Amulet device before the switch to the steerable delivery sheath were used. Adherence to the principles of the Declaration of Helsinki was ascertained and local ethics committee approval was obtained. Data were collected and analyzed from inhospital patient electronic databases. The aim of this study was to analyze periprocedural results and outcomes up to hospital discharge comparing the new Amulet steerable delivery sheath to the previous sheath. Diagnostic criteria for stroke, transient ischemic attack, and bleeding (life threatening, major and minor bleeding) are in adherence to the definitions provided by the Munich consensus document on definitions, endpoints and data collection requirements for LAAC [10].
2.2 Characteristics of Steerable Sheath
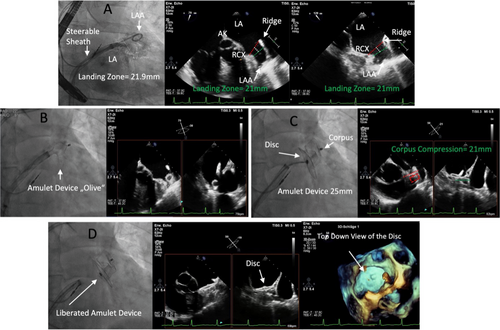
The AMPLATZER STEERABLE DELIVERY SHEATH is specifically designed for LAAC on the time-tested Agilis NxT platforma. With 14 French inner diameter, it fits all Amulet occluder sizes and can be used in all anatomies from simple to challenging. It enables a two-way deflection from 0 to 120° (Figure 1). Bi-directional steering is thought to enable precise device placement to ensure coaxial alignment in the LAA. An auto-lock mechanism provides confidence the sheath will remain in its desired position for the duration of the procedure. Integrated hemostasis valve features simple one-handed operation and minimizes blood loss during catheter introduction and exchange. The steerable sheath could enable controlled maneuverability and responsiveness without the risk of sheath kinking.
2.3 Statistical Analysis
Statistical analysis was performed with Graph Pad Prism Version 9 (GraphPad Software, Inc, San Diego, CA, USA). Categorial variables were analyzed with Chi-Square and Fishers' exact test. Continuous variables were analyzed with Wilcoxon signed-rank test or by paired t-test, where appropriate. Normal distribution was tested with Kolmogorow−Smirnow-test. A two-sided p value of < 0.05 was chosen for statistical significance. Results are reported as mean ± standard deviation (SD). 95% confidence intervals are displayed in Tables 1–3.
| Patient characteristics, mean ± SD or n (%) | All patients | ||
|---|---|---|---|
| Patients with Amulet steerable delivery sheath (95% CI) | Amulet control group (95% CI) | p value | |
| n = 32 | n = 39 | ||
| Age at time of the procedure, years | 80 ± 7.2 (75.8−81.02) | 79 ± 7.6 (75.6−80.6) | 0.8 |
| Gender female, n (%) | 12 (37.5) | 7 (17.9) | 0.1 |
| CHA2DS2-VASc-score, score | 4 ± 1.2 (3.6−4.5) | 4 ± 1.5 (3.8−4.8) | 0.7 |
| HAS-BLED score, score | 3.5 ± 0.1 (3.2−3.8) | 3.8 ± 0.7 (3.6−4.1) | 0.03 |
| Hypertension, n (%) | 27 (84.3) | 33 (82.5) | > 0.9 |
| Diabetes, n (%) | 4 (12.5) | 13 (33) | 0.05 |
| Heart failure, n (%) | 21 (65) | 12 (31) | 0.004 |
| Vascular disease, n (%) | 20 (62) | 24 (61) | > 0.9 |
| History of (ischemic) stroke, n (%) | 5 (15.6) | 6 (15.3) | > 0.9 |
| History of hemorrhagic stroke/ICB, n (%) | 2 (6.2) | 4 (10) | 0.7 |
| History of major bleeding, n (%) | 25 (78) | 34 (87) | 0.3 |
| History of NSAID, long-term antiplatelet medication Clopidogrel additional to OAC, n (%) | 0 | 0 | > 0.9 |
| Abnormal renal function (GFR < 60 mL/min), n (%) | 21 (65) | 25 (64) | > 0.9 |
| GFR (mL/min) | 46.6 ± 4.5 (37.4−55.8) | 55.2 ± 3.7 (47.5-62.8) | 0.2 |
| Dialysis, n (%) | 7 (22) | 1 (3) | 0.01 |
| Patient characteristics, MEAN ± SD or n (%) | All patients | ||
|---|---|---|---|
| Patients with Amulet steerable delivery sheath | Amulet control group | p value | |
| n = 32 (95% CI) | n = 39 (95% CI) | ||
| Procedure | |||
| 34 mm Amulet | 0 | 2 (5.1) | |
| Max LAA diameter landing zone, mm | 28.5 ± 2.1 (9.4−47.5) | ||
| 31 mm Amulet | 5 (16) | 4 (10.2) | |
| Max LAA diameter landing zone, mm | 26.0 ± 2 (22.9−29.5) | 27.5 ± 3.8 (19.6−31.9) | |
| 28 mm Amulet | 7 (21) | 11 (28.2) | |
| Max LAA diameter landing zone, mm | 22 ± 2.4 (19.9−24.3) | 21 ± 2 (19.0−24.1) | |
| 25 mm Amulet | 12 (37.5) | 11 (28.2) | |
| Max LAA diameter landing zone, mm | 20 ± 1.5 (19.3−21.4) | 19 ± 1.7 (18.0−20.6) | |
| 22 mm Amulet | 6 (19) | 7 (18) | |
| Max LAA diameter landing zone, mm | 18 ± 1 (16.4−19.1) | 18 ± 1.6 (16.1−20.2) | |
| 20 mm Amulet | 1 (3) | 1 (2.5) | |
| Max LAA diameter landing zone, mm | 16 ± 0 | 16 ± 0 | |
| 18 mm Amulet | 0 | 1 (2.5) | |
| Max LAA diameter landing zone, mm | 16±1 | ||
| 16 mm Amulet | 1 (3) | 1(2.5) | |
| Max LAA diameter landing zone, mm | 15 ± 0 | 10 ± 0 | |
| Contrast used per procedure, mL | 70 ± 23 (62.3−79.5) | 55 ± 50 (52.5−84.9) | 0.09 |
| Fluoroscopy time, minutes | 12.7 ± 4.6 (11.2−14.6) | 10.2 ± 6.6 (10.5−14.8) | 0.3 |
| Fluoroscopy dose, cGy x cm2 | 2387 ± 12,810 (147−9419) | 2301 ± 3382 (2269−4462) | 0.3 |
| Successful implantation, n (%) | 32 (100) | 39 (100) | > 0.99 |
| Patients with no recaptures, n (%) | 26 (87) | 38 (97) | 0.1 |
| Patients with 1−2 recaptures, n (%) | 6 (19) | 1 (2.5) | 0.1 |
| Patients with > 2 recaptures, n (%) | 0 | 0 | > 0.99 |
| Change to another device size, n (%) | 0 | 2 (5.1) | 0.5 |
| TEE imaging after implantation | |||
| Device changed position after release, n (%) | 0 | 0 | > 0.99 |
| Complete seal, n (%) | 31 (97) | 37 (95) | > 0.99 |
| If no: jet < 3 mm, n (%) | 1 (3.1) | 2 (5.1) | > 0.99 |
| Periprocedural events | |||
| Duration hospital stay, days | 2 ± 5.4 (2.9−6.9) | 4 ± 7.4 (4.0−8.8) | 0.7 |
| Pericardial effusion, n (%) | 1 (3.1) | 1 (2.5) | > 0.99 |
| Pericardial effusion with tamponade, n (%) | 0 | 1 (2.5) | > 0.99 |
| Device embolism, n (%) | 0 | 0 | > 0.99 |
| Air embolism (coronary), n (%) | 0 | 0 | > 0.99 |
| Vascular access complication, n (%) | 0 | 0 | > 0.99 |
| Procedural major bleeding, n (%) | 0 | 0 | > 0.99 |
| Access-site infection, n (%) | 0 | 0 | > 0.99 |
| Stroke periprocedural, n (%) | 0 | 0 | > 0.99 |
| Death procedure-related, n (%) | 0 | 0 | > 0.99 |
| Postprocedural drug regiment up to the first follow-up | |||
| DAPT, n (%) | 32 (100) | 39 (100) | > 0.99 |
| Peri-device leak (PDL) on 1st FU | 1 | 3 | 0.62 |
| Device-related thrombus on 1st FU | 0 | 2 | 0.49 |
| LAA-morphology | |||
| Chickenwing | 11 | 9 | |
| Cauliflower | 15 | 28 | |
| Windsock | 3 | 2 | |
| Cactus | 3 | 0 | |
| Patient characteristics, mean ± SD or n (%) | Learning curve | |||
|---|---|---|---|---|
Patients with Amulet steerable delivery sheath first 3 M n = 20 (95% CI) |
Patients with Amulet steerable delivery sheath > 3 M n = 12 (95% CI) |
Amulet control group n = 39 (95% CI) |
p value ANOVA comparison to the control group |
|
| Contrast used per procedure, mL | 67.7 ± 19 (58.5−76.9) | 76.8 ± 29.4 (57.0−96.5) | 55 ± 50 (52.5−84.9) | 0.2 |
| Fluoroscopy time | 13.2 ± 3.7 (11.0−14.7) | 12.2 ± 6 (9.0−17.1) | 10 ± 6 (10.5−14.8) | 0.5 |
| Fluoroscopy dose, cGy x cm2 | 2447 ± 1033 (1876−2872) | 1277 ± 21,154 (5668−22,754) | 2301 ± 3382 (2269−4462) | 0.1 |
3 Results
Baseline characteristics were comparable between the groups (Table 1) (steerable sheath vs. control group: age: 80 ± 7.2 vs. 79 ± 7.6 years mean ± SD, p = 0.8; female gender 37.5 vs. 17.9%, p = 0.1). The CHA2DS2Vasc-Score was comparable (steerable sheath vs. control group: 4 ± 1.2 vs. 4 ± 1.5, p = 0.1). We observed differences in the HAS-Bled-Score (steerable sheath vs. control group: 3.5 ± 0.1 vs. 3.8 ± 0.7, p = 0.03*), history of heart failure (steerable sheath vs. control group: 65 vs. 31%, p = 0.004*) and renal failure with dialysis (steerable sheath vs. control group: 22 vs. 3%, p = 0.01*). Implantation (Figure 1) was successful in all patients (100%) in both groups with implantation of adequate device sizes according to the instructions for use (Table 2).
The two dominant morphologies were chickenwing and cauliflower in both groups.
Contrast use and fluoroscopy time were numerically higher in the steerable sheath group (steerable sheath vs. control group: contrast use 70 ± 23 vs. 55 ± 50 mL, p = 0.09, fluoroscopy time 12.7 ± 4.6 vs. 10.2 ± 6.6 min, p = 0.3). We assumed a learning curve and analyzed contrast use and fluoroscopy time during the first 3 months with the steerable sheath and > 3 months. Fluoroscopy time and dose decreased after 3 months, but the contrast use remained unchanged (Table 3). However, we could neither see a decrease in procedure time during the time course studied nor between the different interventionalists involved in LAAC.
Complete sealing rate was high in both groups (steerable sheath vs. control group 97 vs. 95%, p > 0.9). We observed no change to another device in the steerable sheath group as compared to 2 changes to another size in the control group (Table 2) (0 vs. 5.1%, p = 0.5). The pericardial effusion rate was low (steerable sheath vs. control group 3.1 vs. 2.5%, p > 0.9), with one observed pericardial tamponade in the control group (steerable sheath vs. control group 0 vs. 2.5%, p > 0.9). Periprocedural complication rate was low, without any periprocedural stroke, vascular complications, or death in both cohorts (Table 2). One patient in the steerable sheath group had an initial leak during the index procedure < 3 mm. In the control group two initial leaks, both < 3 mm, were detected (Table 2). PDL could be observed with transesophageal echocardiography in one patient in the steerable sheath group and in three patients in the control group on the first follow-up after 3 months. In the steerable sheath group, the leak was 3−5 mm. In the control group all leaks were <3 mm. Due to the small size of the leaks, no additional measures were considered. DRT was only observed in the control group (two patients) on first follow-up after 3 months (Table 2). In both patients, antithrombotic regimen was changed to anticoagulation. One patient was initially treated with low-molecular weight heparin due to NOAK intolerance. Later-on antithrombotic regimen was changed to phenprocoumon which finally led to disappearance of DRT. The other patient received apixaban which also resolved DRT.
4 Discussion
We are among the first reporting systematic data of the steerable delivery sheath specifically designed for optimized LAAC. The success rate of LAAC is high, but interventionalists are still facing some challenging LAA anatomies that may increase the risk of suboptimal results. Amabile et al could demonstrate improved results of LAAC, that is, significantly lower incidence of residual patent LAA, PDL and off-axis device position in patients with severely enlarged left atrium when the steerable sheath was used [11].
5 What Options Does the New Steerable Delivery Sheath Offer
Four main LAA anatomies have been described in the literature: Cactus, Chicken Wing, Windsock and Cauliflower LAA [12]. The ideal device should be (1) easy to use, (2) be able to adapt to a large variety of LAA anatomies to enable complete LAA closure, protection from device-related thrombus and pericardial effusion and (3) offers safety regarding device related complications and protection from stroke. No significant difference regarding implantation success and complication rate have been reported for the four different anatomies [13]. In contrast, in atypical LAA-anatomies implantation success has been reported to be lower [13]. The steerable delivery sheath might overcome difficulties in LAAC in atypical LAA anatomies thereby even increasing implantation success rate. We observed a high success rate in LAAC. Corresponding to the study of Amabile et al we could also see numerically more patients with PDL in the control group than in the steerable delivery sheath group. DRT was only observed in the control group which was in line with the results reported by Amabile et al. [11]. Since PDL are related to operator experience [14], and in our study only experienced operators were involved, this might explain why there were no significant differences between both groups. Furthermore, analysis of PROTECT-AF and PREVAIL demonstrated, that a substantial number of PDL decreased in size or even disappeared over time [6]. Clinical data (711 patients, 237 with DRT and 474 without) underline the importance of DRT since its presence was associated with a higher incidence of an endpoint comprising death, stroke or systemic embolization, mainly driven by ischemic strokes after LAAC [15]. This was further supported by a meta-analysis comprising 7827 patients [16] which revealed a significantly elevated stroke risk when DRT was identified (11% risk in DRT patients, 2.9% risk in non-DRT patients [16]. In addition, an association of DRT with residual peri device leak has been proven [17]. Also, renal insufficiency, was shown to be an important DRT predictor [15].
6 Learning Curve and Results for LAAC
Data from registry-based research trials suggest a learning curve for LAAC. Jung et al. used a nationwide database (Vizient Clinical Database comprising > 400 centers in the US with 13.651 LAAC procedures) to address the question: how is the relationship between the learning curve, vascular complications and clinical outcomes in patients undergoing interventional LAAC? The primary endpoint was a composite of mortality, sternotomy/pericardiectomy, major vascular complications and stroke. Statistical significance regarding the primary endpoint was achieved at a cut-off value of 30 patients per hospital [18]. In addition major adverse events were reported to be as low as 1.4% [18]. In our study, implementation of a new steerable delivery sheath into routine clinical practice was not associated with an increase of adverse events. With respect to parameters of the implantation procedure, Ledwoch et al. showed an increase in implantation success for an increasing number of procedures performed [19]. Notably, procedure time, fluoroscopy time and contrast volume decreased with an increasing number of patients treated with LAAC [19]. Similar results were reported by Cruz-Gonzales et al. who analyzed LAAC implantation success and complications with a focus on operator experience and two different devices (Amplatzer cardiac plug and Watchman device) [20]. Here, a decrease in cardiac complications with increasing operator experience was reported. Interestingly, implantation of another device (Watchman) later-on during the study was not associated with an increase of complications [20] suggesting that adequately trained interventionalists are able to rapidly accommodate to new material and patient safety is maintained. We could also see a tendency toward a decrease in procedure- and fluoroscopy time before and after 3 months of experience with the steerable delivery sheath. However, no statistical significance was reached, and the amount of contrast media stayed unchanged over time. Also, we could neither see a decrease in procedure time during the time course studied nor between the different interventionalists involved in LAAC.
7 Limitations
The main limitations of our study are the retrospective character, and a small number of patients included. Also, differences in baseline characteristics could be observed. Furthermore, only patients receiving an Amulet device were analyzed. In addition, the manufacturer of the steerable delivery sheath has voluntarily recalled the sheath due to potential safety issues regarding air embolism, LAA perforation and tamponade. However, this complication was not observed in our patient cohort. An improved version of the steerable sheath is awaited soon and should also address the problems of stiffness and French size of the sheath, as some experienced LAAC users included us have judged the steerable sheath as too stiff and bulky. During the study there were only limited complex anatomies in our patient cohort, mainly cauliflower morphology in both groups. However, in patients with retrobend chicken-wing LAA, low LAA position, pronounced posterior or anterior axis or complex transseptal puncture the steerable sheath might be especially helpful.
8 Conclusion
LAAC with the Amplatzer Amulet Steerable Delivery Sheath is feasible and safe. Fluoroscopy time and dose suggest a learning curve with the new sheath. No increases in adverse events could be observed with the new sheath. The use of a steerable sheath during a LAAC procedure could potentially increase the success rate and decrease the complication rate (leaks, device embolizations or device related thrombi) in selected patients thanks to a more coaxial approach of device deployment.
Especially in patients with retrobend chicken-wing LAA, low LAA position, pronounced posterior or anterior axis or complex transseptal puncture the steerable sheath might be helpful.
Ethics Statement
Regarding our study, adherence to the principles of the Declaration of Helsinki was ascertained and local ethics committee approval was obtained. Data were collected and analyzed from inhospital patient electronic databases. No particular research funding was obtained. Matthias Mezger, Christina Paitazoglou and Thomas Stiermaier further declare that they have no conflict of interest. Ingo Eitel received speaker honoraria from ABBOTT and Boston Scientific and Study support from ABBOTT.
Conflicts of Interest
I.E. received speaker honoraria from ABBOTT and Boston Scientific and Study support from ABBOTT. The other authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




