Coronary Plaque Characteristics Assessed by Optical Coherence Tomography and Plasma Lipoprotein(a) Levels in Patients With Acute Coronary Syndrome
Part of this work has been accepted as a moderated abstract at the TCT Conference 2024.
Carlo Di Mario and Alessio Mattesini are co-senior authors.
ABSTRACT
Background
Lipoprotein(a) [Lp(a)] is associated with increased cardiovascular risk, but its influence on plaque characteristics at optical coherence tomography (OCT) evaluation is not fully understood.
Aims
This study seeks to explore the impact of Lp(a) levels on plaque morphology as assessed by OCT in a very high-risk subset of patients.
Methods
Consecutive patients admitted for acute coronary syndrome (ACS) and undergoing OCT-guided percutaneous coronary intervention (PCI) at a large tertiary care center between 2019 and 2022 were deemed eligible for the current analysis. The overall population was categorized into two subgroups according to baseline Lp(a) levels: (1) lower Lp(a) (Lp(a) ≤ 300 mg/L) and (2) elevated Lp(a) (Lp(a) 300 mg/L). Predictors of lipid-rich plaques were identified using multivariable logistic regression with stepwise selection of candidate covariates.
Results
A total of 202 patients were included in this study. OCT findings revealed that patients with elevated Lp(a) had a higher prevalence of lipid-rich plaques, a significantly greater mean lipid arc, along with increased macrophage infiltration and thin-cap fibroatheroma (TCFA). In contrast, calcific plaque prevalence was higher in the lower Lp(a) group. Multivariable regression analysis identified low-density lipoprotein cholesterol ≥ 70 mg/dL, and elevated Lp(a) as strong predictors of lipid-rich plaques at OCT.
Conclusion
In this observational study including ACS patients undergoing OCT-guided PCI, those with elevated Lp(a) levels exhibited a higher prevalence of lipid-rich plaques, increased macrophage infiltration, and TCFA, thereby indicating a more vulnerable plaque phenotype. Additionally, elevated Lp(a) levels and LDL-C levels ≥ 70 mg/dL were each independently associated with lipid enrichment of coronary plaques. These findings suggest Lp(a), beyond other well-known risk factors, as a key marker for risk stratification, potentially informing optimal medical management strategies.
Abbreviations
-
- ACS
-
- acute coronary syndrome
-
- CI
-
- confidence interval
-
- LDL-C
-
- low-density lipoprotein cholesterol
-
- Lp(a)
-
- lipoprotein(a)
-
- OCT
-
- optical coherence tomography
-
- OR
-
- odds ratio
-
- PCI
-
- percutaneous coronary intervention
-
- TCFA
-
- thin-cap fibroatheroma
1 Introduction
Acute coronary syndromes (ACS) are a leading cause of morbidity and mortality worldwide, claiming for approximately 9 million deaths annually [1-3]. Interestingly, despite advances in therapeutic interventions and secondary prevention strategies, such as high-intensity statin therapy or anti-inflammatory drugs, patients with ACS continue to face a significant residual cardiovascular risk, leaving them vulnerable to future cardiovascular events [4, 5].
Extensive evidence has shown that the progression and destabilization of atherosclerotic plaques are complex pathological processes involving numerous factors beyond low-density lipoprotein cholesterol (LDL-C) [6, 7]. As a result, the identification of novel proatherogenic risk factors, beyond traditional ones, has been and currently is a key research focus.
Among inflammatory markers, lipoprotein(a) (Lp(a)) has emerged as an independent and causal risk factor for atherosclerotic cardiovascular disease [8]. Lp(a) is an apoB100-containing lipoprotein bound to apolipoprotein(a). Its levels are genetically determined, show slight variation across the lifespan, and are unaffected by lifestyle or other biomarkers [9, 10]. The hypothesis suggests that Lp(a) may contribute to atherosclerosis through its lipoprotein component and to thrombosis via the plasminogen-like apo(a) part. Additionally, it may play distinct proinflammatory effects due to the generation of oxidized phospholipids, which are key triggers for the activation of the NLRP3 inflammasome and subsequent cytokine cascade [11, 12]. For these reasons, it has been explored and is currently under investigation as a target for primary and secondary prevention, including emerging therapies such as PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors and antisense oligonucleotides directly targeting apolipoprotein(a) [13, 14]. Nevertheless, the association of Lp(a) with specific plaque characteristics at intravascular imaging, has not been fully understood. Therefore, we aimed to clarify the influence of Lp(a) levels on plaque morphology as assessed by OCT in the very high-risk subset of patients hospitalized for ACS.
2 Methods
2.1 Study Design and Patient Population
Patients with an admission diagnosis of ACS and undergoing OCT-guided PCI at a large-volume tertiary-care center in Italy (Careggi University Hospital, Florence) between January 2019 and December 2022 were prospectively enrolled in the institutional OCT database and considered for inclusion in the current retrospective analysis. The diagnosis of ACS was established according to the 2023 European Society of Cardiology Guidelines [15]. Patients presenting cardiogenic shock or cardiac arrest at admission, poor OCT quality runs, or missing baseline Lp(a) measurements were excluded.
Data on baseline, procedural characteristics, and medications were prospectively obtained from electronic health records. Each patient provided informed consent for the anonymous processing of their information. PCI procedures followed the standard protocols, with the treating physician's choice of stent and technique. The medical management, including the discharge antithrombotic therapy, was customized according to the clinical presentation, patient comorbidities, and physician preferences.
Blood samples were collected according to routine clinical practice before the index procedure. The laboratory evaluation included a comprehensive lipid profile, measuring total cholesterol, LDL-C, high-density lipoprotein cholesterol, triglycerides, and Lp(a). Lp(a) values were determined using the ELISA technique with monoclonal antibodies based on the immunoturbidimetric principle targeting the apo(a) component of Lp(a) in a fasting state, as its levels can be falsely elevated after meals.
The overall population was divided into two groups based on Lp(a) levels using the threshold adopted by our institutional laboratory: patients with Lp(a) < 300 mg/L were classified as having low levels, while those with Lp(a) ≥ 300 mg/L were categorized as having elevated levels.
The study adhered to the principles outlined in the World Medical Association Declaration of Helsinki, International Organization for Standardization Guidelines, and Good Clinical Practice Guidelines [16].
2.2 OCT Analysis
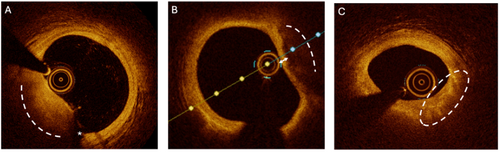
Intravascular OCT imaging was performed before and after PCI using a frequency domain OCT system (ILUMIEN OPTIS, St. Jude Medical/Abbott, St. Paul, MN, USA) and a Dragonfly catheter (Lightlab Imaging Inc., Westford, MA, USA). All pullbacks were analyzed offline, accurately examining plaque characteristics in pre-PCI pullbacks and assessing stent deployment results after PCI. The standardized procedure was as follows: after placing the 6 or 7 Fr guiding catheter and wiring the culprit lesion, the OCT catheter was advanced distally to the target lesion. Once the lesion length and minimal lumen area (MLA) were identified, a qualitative assessment of the coronary lesion was performed at the MLA. The following measurements were recorded: mean lipid arc and lipid length, mean calcium arc and calcium length, presence of macrophages, cholesterol crystals, intimal vasculature, and thin-cap fibroatheroma (TCFA). Based on these measurements, coronary plaques were classified as lipid-rich, fibrous, or calcific. In cases with borderline characteristics, we defined the plaque according to the predominant pattern. TCFA was defined as a fibrous cap < 65 microns overlying a large lipid-rich necrotic core [17]. Examples of plaque characteristics observed during OCT evaluation are provided in Figure 1.
2.3 Statistical Analysis
Continuous variables were presented as means standard deviation and compared using Student's t test or the Mann–Whitney U test for variables without normal distribution. Categorical variables were reported as percentages and frequencies, and the comparisons were performed with the χ2 test or Fisher exact test. Univariate and multivariate regression analyses were computed to investigate the associations of Lp(a) and characteristics of coronary atherosclerosis. Predictors of lipid-rich plaques were identified using multivariable logistic regression with stepwise selection of candidate covariates. Odds ratio (OR) and 95% confidence interval (CI) were calculated and reported. A p < 0.05 was considered statistically significant. All data were analyzed using the SPSS version 28.0 software (SPSS Inc., Chicago, IL, USA).
3 Results
3.1 Baseline Clinical and Procedural Characteristics
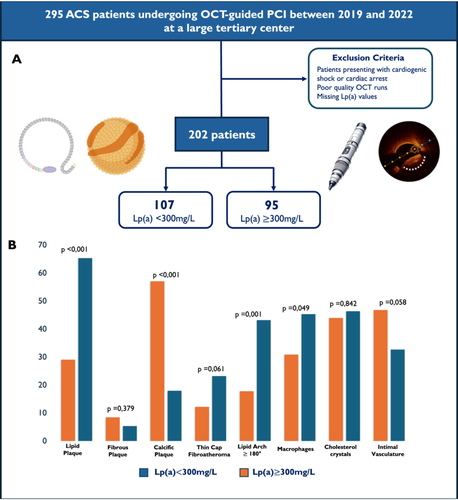
After considering the selection criteria, 202 of the 295 ACS patients who underwent OCT-guided PCI at our catheterization laboratory were included in the analysis (19.8% female, mean age 67.17 ± 11.27 years). Among these, 107 (52.9%) had low Lp(a) levels, while 95 (47.1%) exhibited elevated Lp(a) values. Baseline characteristics are fully displayed in Table 1. Patients with elevated Lp(a) were slightly younger, with a mean age of 65.55 ± 12.61 years and most commonly presented with NSTEMI or unstable angina. The prevalence of cardiovascular risk factors and comorbidities was similar between the two groups, with no relevant differences observed. However, at laboratory evaluation, patients with Lp(a) ≥ 300 mg/L demonstrated a more altered lipid profile, showing higher mean total cholesterol (163.19 ± 65.88 vs. 142.77 ± 37.69 mg/dL in the low Lp(a) group) and mean LDL-C levels (104.27 ± 62.65 vs. 82.62 ± 35.16 mg/dL in the low Lp(a) group).
Lp(a) < 300 mg/L N = 107 (52.9%) |
Lp(a) 300 mg/L N = 95 (47.1%) |
p value | |
|---|---|---|---|
| Patient demographics | |||
| Age, years | 68.79 ± 9.94 | 65.55 ± 12.61 | 0.043 |
| BMI, kg/m2 | 25.96 ± 4.79 | 25.51 ± 4.95 | 0.513 |
| Female sex | 18 (16.8%) | 22 (23.16%) | 0.342 |
| Medical history | |||
| Current smoker | 38 (35.5%) | 28 (29.5%) | < 0.001 |
| Former smoker | 25 (23.4%) | 23 (24.2%) | 0.658 |
| Family history of CAD | 39 (36.4%) | 36 (37.9%) | 0.947 |
| Diabetes mellitus | 32 (29.9%) | 28 (29.5%) | 0.946 |
| Hypertension | 76 (71.0%) | 69 (72.6%) | 0.923 |
| Hyperlipidemia | 25 (23.4%) | 23 (24.2%) | 0.980 |
| Peripheral artery disease | 7 (6.5%) | 3 (3.2%) | 0.434 |
| Cerebrovascular disease | 2 (1.9%) | 0 (0%) | 0.086 |
| COPD | 7 (6.5%) | 5 (5.3%) | 0.932 |
| Chronic kidney disease | 4 (3.7%) | 9 (9.5%) | 0.136 |
| Prior PCI | 18 (16.8%) | 15 (15.8%) | 0.967 |
| Prior MI | 15 (14.0%) | 19 (20.0%) | 0.705 |
| Prior CABG | 2 (1.9%) | 0 (0%) | 0.530 |
| Laboratory | |||
| Glucose mg/dL | 104.01 ± 36.30 | 119.43 ± 70.82 | 0.050 |
| HbA1c mmol/mol | 42.65 ± 5.18 | 44.00 ± 10.22 | 0.229 |
| Serum creatinine, mg/dL | 0.9 ± 0.20 | 1.05 ± 0.7 | 0.069 |
| Total cholesterol, mg/dL | 142.77 ± 37.69 | 163.20 ± 65.88 | 0.007 |
| Low-density lipoprotein, mg/dL | 82.6 ± 35.2 | 104.3 ± 62.6 | 0.002 |
| High-density lipoprotein, mg/dL | 45.6 ± 14.2 | 47.02 ± 11.7 | 0.443 |
| Triglycerides, mg/dL | 108.5 ± 41.1 | 110.5 ± 49.1 | 0.747 |
| Lp(a), mg/L | 98.1 ± 8.9 | 424.6 ± 11.2 | < 0.001 |
| Clinical presentation | 0.440 | ||
| STEMI | 20 (18.7%) | 14 (14.7%) | |
| NSTEMI | 44 (41.1%) | 40 (42.1%) | |
| Unstable angina | 43 (40.2%) | 41 (43.2%) | |
| Admission medication | |||
| Aspirin | 66 (61.7%) | 61 (64.2%) | 0.708 |
| DAPT | 58 (54.1%) | 52 (54.7%) | 0.782 |
| Statins | 73 (68.2%) | 60 (63.2%) | 0.646 |
| Beta-blockers | 66 (61.7%) | 54 (56.8%) | 0.578 |
| Ace-inhibitors | 45 (42.1%) | 45 (47.4%) | 0.538 |
- Abbreviations: BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DAPT, dual antiplatelet therapy; Lp(a), lipoprotein(a); MI, myocardial infarction; PCI, percutaneous coronary intervention.
As shown in Table 2, no significant differences in procedural characteristics were seen. Most of the procedures were performed via radial access (93.5% and 91.6%, in patients with low and elevated Lp(a) respectively); a large proportion of patients presented with single vessel disease, while around 30% exhibited two-vessel involvement; a consistent proportion of patients showed in-stent restenosis, bifurcation involvement, and moderate/severe calcification in both groups. No significant differences were detected in peri-procedural and in-hospital complications as displayed in Supporting Information S1: Table S1.
Lp(a) < 300 mg/L N = 107 (52.9%) |
Lp(a) 300 mg/L N = 95 (47.1%) |
p value | |
|---|---|---|---|
| Procedural characteristics | |||
| Radial access | 100 (93.5%) | 85 (89.4%) | 0.461 |
| Femoral access | 7 (6.5%) | 10 (10.6%) | 0.309 |
| Lesions and PCI characteristics | |||
| Left main | 16 (14.9%) | 12 (12.6%) | 0.785 |
| CAD 1 vessel | 53 (49.5%) | 48 (50.5%) | 0.077 |
| CAD 2 vessels | 31 (28.9%) | 36 (37.9%) | 0.077 |
| CAD 3 vessels | 18 (16.8%) | 6 (6.3%) | 0.077 |
| Bifurcation | 12 (11.2%) | 10 (10.5%) | 0.014 |
| Chronic total occlusion | 1 (0.9%) | 0 (0%) | 0.014 |
| Moderate/severe calcification | 18 (16.8%) | 5 (5.3%) | 0.014 |
| Intrastent restenosis | 11 (10.3%) | 12 (12.6%) | 0.600 |
| Number OCT identified lesion | |||
| 1 | 90 (84.1%) | 73 (76.8%) | 0.468 |
| 2 | 15 (14.0%) | 18 (18.9%) | 0.468 |
| 3 | 2 (1.9%) | 3 (3.2%) | 0.468 |
| Treated with PCI | 91 (85%) | 78 (82.0%) | 0.582 |
| Predilatation | 69 (64.5%) | 63 (66.3%) | 0.837 |
| Single stent PCI | 48 (44.9%) | 38 (40.0%) | 0.590 |
| Multiple stent PCI | 32 (29.9%) | 26 (27.4%) | 0.590 |
| PCI with drug-eluting balloon | 7 (6.5%) | 11 (11.6%) | 0.467 |
| Fluoroscopy time (min) | 20.4 ± 11.4 | 18.4 ± 10.7 | 0.209 |
| Contrast (vol) | 173.6 ± 60.4 | 168.0 ± 63.8 | 0.529 |
| OCT assessment of the culprit lesion | |||
| Plaque rupture | 26 (24.3%) | 30 (31.6%) | 0.474 |
| Plaque erosion | 14 (13.1%) | 20 (21.0%) | 0.277 |
| Thrombus | 4 (3.7%) | 6 (6.3%) | 0.757 |
| Prevalent plaque type | |||
| Lipid plaque | 31 (29.0%) | 62 (65.3%) | 0.002 |
| Fibrous plaque | 9 (8.4%) | 5 (5.3%) | 0.379 |
| Calcific plaque | 61 (57.0%) | 17 (17.9%) | < 0.001 |
| Minimum lumen area, mm2 | 2.8 ± 1.37 | 2.98 ± 1.90 | 0.507 |
| Mean lipid arc (°) | 88.45 ± 11.6 | 146.14 ± 12.42 | < 0.001 |
| Mean lipid length (mm) | 6.21 ± 1.02 | 11.24 ± 1.16 | < 0.001 |
| Lipidic arc > 180° | 19 (17.8%) | 41 (43.2%) | 0.001 |
| TCFA | 13 (12.2%) | 22 (23.2%) | 0.061 |
| Presence of macrophage | 33 (30.8%) | 43 (45.3%) | 0.049 |
| Cholesterol crystals | 47 (43.9%) | 44 (46.3%) | 0.842 |
| Microvasculature | 50 (46.7%) | 31 (32.6%) | 0.058 |
| Mean calcium arc (°) | 139.41 ± 12.39 | 68.82 ± 10.26 | < 0.001 |
| Calc length > 0.5 mm | 27 (65.9%) | 14 (34.1%) | < 0.001 |
| Calc length > 1 mm | 41 (68.3%) | 19 (31.7%) | < 0.001 |
| Fujino score | |||
| Fujino score 1 | 6 (5.6%) | 3 (3.2%) | < 0.001 |
| Fujino score 2 | 14 (13.1%) | 3 (3.2%) | < 0.001 |
| Fujino score 3 | 5 (4.7%) | 2 (2.1%) | < 0.001 |
| Fujino score 4 | 27 (25.2%) | 11 (11.6%) | < 0.001 |
- Abbreviations: CAD, coronary artery disease; LM, left main; Lp(a), lipoprotein(a); OCT, optical coherence tomography; PCI, percutaneous coronary intervention.
3.2 Qualitative and Quantitative OCT Findings
At the pre-PCI OCT examination, the prevalence of plaque rupture or plaque erosion was similar in the two cohorts. Patients with elevated Lp(a) levels demonstrated a higher prevalence of lipidic lesions (n = 62, 65.5%) compared to those with low Lp(a) levels, primarily due to a greater mean lipid arc and mean lipid core length. In contrast, patients with Lp(a) < 300 mg/L exhibited a greater prevalence of calcific lesions (n = 61, 57%), characterized by a larger mean calcium arc, a more frequent longitudinal calcium length > 1 mm, and a greater calcium thickness. Also, these patients had a Fujino score > 1 in 42.9% of cases, compared to only 16.8% in those with elevated Lp(a) levels. TCFA and macrophages were more frequently observed in patients with elevated Lp(a) levels. In contrast, no significant differences between the groups regarding cholesterol crystals and intimal vasculature were found.
3.3 Univariate and Multivariate Analysis
The results of the univariate and multivariate logistic regression analyses identifying factors associated with the presence of lipid-rich plaques, as assessed by OCT, are summarized in Table 3. After stepwise selection of covariates in the univariate analysis, some factors included in the multivariate model remained significantly associated with lipid-rich plaques. Notably, elevated LDL-C levels (≥ 70 mg/dL) as well as Lp(a) levels ≥ 300 mg/L emerged as strong predictors of an increased risk of lipid-rich plaques (OR 2.39, 95% CI 1.22–4.65, p = 0.010 and OR 4.41, 95% CI 2.35–8.28, p < 0.001, respectively). In contrast, age > 65 years was linked to a reduced likelihood of detecting lipid-rich plaques.
Univariate analysis odds ratio (95% CI) |
p value | Multivariate analysis odds ratio (95% CI) |
p value | |
|---|---|---|---|---|
| Variables | ||||
| Age > 65 years* | 0.44 (0.25– 0.79) | 0.006 | 0.53 (0.28–1.01) | 0.057 |
| Female sex | 0.65 (0.32–1.33) | 0.243 | ||
| Smoking habit | 0.79 (0.55–1.12) | 0.195 | ||
| Family history of CAD | 0.93 (0.52–1.66) | 0.809 | ||
| Hypertension | 0.85 (0.46–1.57) | 0.599 | ||
| Hyperlipidemia | 0.87 (0.70–1.08) | 0.208 | ||
| Serum creatinine, mg/dL | 1.90 (0.76–4.77) | 0.169 | ||
| Low-density lipoprotein ≥ 70 mg/dL | 2.6 (1.4–4.84) | <0.002 | 2.39 (1.22–4.65) | 0.010 |
| Lp(a), mg/L* | 4.60 (2.50–8.34) | <0.001 | 4.41 (2.35–8.28) | <0.001 |
| No statin usage | 1.53 (0.80–2.90) | 0.197 | ||
- Abbreviations: CAD, coronary artery disease; CI, confidence interval; Lp(a), lipoprotein(a); OR, odds ratio.
4 Discussion
-
Patients with elevated Lp(a) levels exhibited a higher prevalence of lipid-rich plaques, along with increased macrophage infiltration and TCFA, while those with lower Lp(a) levels had a higher prevalence of calcific plaques (Central Illustration 1);
-
Lp(a) ≥ 300 mg/L and LDL-C levels ≥ 70 mg/dL were both strongly linked to the presence of lipid-rich plaques at OCT assessment;
-
Patients older than 65 years had a lower likelihood of presenting with lipid-rich plaques at OCT evaluation.
Several studies have demonstrated the association between elevated Lp(a) levels and the extent of coronary artery disease [18, 19]; however, limited evidence is available regarding the relationship between Lp(a) and atherosclerotic plaque phenotype detected at intravascular imaging. To the best of our knowledge, this is the largest study to offer a comprehensive OCT assessment of culprit plaque characteristics in a high-risk subset of patients, such as those undergoing PCI for ACS, stratified by Lp(a) levels. Our results align with previous literature based on different imaging techniques such as coronary computed tomography angiography or intravascular ultrasound which have suggested a role of Lp(a) in plaque progression and destabilization [20, 21]. Among these, Niccoli et al. showed a predominance of lipid-rich plaques in patients with high Lp(a) levels in a small cohort of 51 ACS patients undergoing OCT-guided coronary angiography [22]. Similarly, a post hoc analysis of 6 randomized trials identified Lp(a) as a reliable predictor of plaque atheroma volume detected with intravascular ultrasound, supporting its proatherogenic contribution to cardiovascular risk [23].
Interestingly, our analysis revealed a predominance of vulnerable plaque features, such as macrophage infiltration and TCFA, in subjects with Lp(a) levels ≥ 300 mg/L, further reinforcing the evidence of Lp(a)'s proinflammatory effects alongside its established proatherogenic role. This finding is consistent with a large translational study that demonstrated a strong association between elevated Lp(a) and increased arterial wall inflammation, as measured by multiple imaging modalities, including magnetic resonance, positron emission tomography/computed tomography, and single photon emission computed tomography/computed tomography, along with enhanced expression of proinflammatory genes in monocytes [24].
These combined proatherogenic and inflammatory effects, which contribute to both lipid-plaque enrichment and increased plaque vulnerability, might stem from the dual composition of Lp(a): a single molecule of apolipolipoprotein B-100 and the plasminogen-like apo(a) moiety [10]. Indeed, apo(B) may be the primary responsible for driving atherosclerosis progression due to its similarity to LDL [25]. On the other hand, oxidized phospholipids, present either in the lipid phase of Lp(a) or bound to apo(a), play a critical role in promoting monocyte trafficking by triggering cytokine production and facilitating monocyte entry into the vessel wall through monocyte chemoattractant protein-1, which is directly exposed to Lp(a)'s surface [26, 27].
Finally, we observed a negative association between older age and lipid-rich plaques, along with a higher prevalence of calcified plaques in patients with low Lp(a) levels. This contrasts with existing evidence suggesting a procalcific effect of Lp(a), revealing a potential inconsistency in our analysis that warrants further investigation in larger cohorts [28, 29]. A potential explanation for both these findings could be the slightly older age observed in patients with low Lp(a) levels along with the more frequent use of statin therapy in this cohort, both of which are well-known contributors to the calcification process [30, 31].
Overall our results have significant implications for improving diagnostic accuracy and guiding pharmacological treatment. Traditionally, Lp(a) has been regarded as a secondary target in patients experiencing cardiovascular events despite aggressive lipid-lowering therapies. However, beyond the well-established linear relationship between elevated Lp(a) and ASCVD risk, a high rate of risk reclassification has been shown in patients with very high Lp(a) levels, even in the absence of ASCVD identifying individuals who could benefit from early treatment [32]. Therefore, Lp(a) should be integrated into risk stratification and as a therapeutic target—not only at the time of PCI when patients are already at very high risk and exhibit more unstable plaques as in our analysis, but also during early screening to implement primary preventive measures. So far, studies showing potential benefits from Lp(a) apheresis have fueled ongoing research into specific Lp(a)-targeting interventions, such as antisense oligonucleotides, small interfering RNA-based therapies, and oral agents [14, 33, 34]. Further research is needed to clarify Lp(a)'s contribution to residual cardiovascular risk—both alone and in combination with other biomarkers—and to determine how best to incorporate it into widely used risk prediction and stratification tools. Additionally, future studies should refine the enrollment criteria to include not only high-risk patients but also those in earlier stages, and explore Lp(a) as a potential target for primary prevention.
5 Limitations
Our study has several limitations that should be considered for a correct interpretation of the results. First, its observational nature, combined with the relatively small sample size, data collection from a single PCI center and the absence of a standardized threshold for Lp(a) may limit the external validity of our findings. Second, despite being reviewed by two independent expert operators, the interpretation of OCT runs remains subjective, introducing potential bias. Third, the OCT analysis was confined to the culprit vessel, limiting the generalizability of our conclusions to other coronary vessels. Moreover, pre-PCI OCT was often performed after vessel recanalization with thrombectomy or thrombus aspiration and/or gentle balloon dilatation, which may have introduced bias in the accurate assessment of some culprit plaque features, including plaque rupture, plaque erosion, and thrombus. Finally, we did not collect detailed data on medications other than the use of statins, such as their dosage, duration, and type, which could have provided additional insights given their influence on plaque progression and calcification.
6 Conclusion
In this real-world cohort of ACS patients undergoing OCT-guided PCI, those with elevated Lp(a) levels (≥ 300 mg/L) exhibited a higher prevalence of lipid-rich plaques, increased macrophage infiltration, and thin-cap fibroatheroma, thereby indicating a more vulnerable plaque phenotype. Additionally, elevated Lp(a) levels and LDL-C levels ≥ 70 mg/dL were each independently associated with lipid-rich plaques. This underscores the significance of considering Lp(a) alongside LDL-C in improving risk stratification and guiding personalized treatment strategies to optimize outcomes in this high-risk patient population.
Acknowledgments
The authors have nothing to report. Open access publishing facilitated by Universita degli Studi di Firenze, as part of the Wiley - CRUI-CARE agreement.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.




