Endovascular Treatment of Malfunctioning Dialysis Fistulas: A Multicenter Retrospective Analysis Comparing Transradial and Conventional Transvenous Access
ABSTRACT
Background
Venous outflow is the favored access for endovascular management of dialysis fistulas. However, transradial access (TRA) offers advantages in specific clinical scenarios. The study aims to compare the efficacy, feasibility, and safety of TRA and transvenous access (TVA) in the endovascular management of malfunctioning dialysis fistulas, addressing the existing gap in comprehensive literature.
Methods
A retrospective multi-center analysis included prospectively collected data (January 2021–November 2023) from patients undergoing endovascular management of malfunctioning dialysis fistulas with TRA. Control groups comprised patients with TVA.
Results
Of 206 patients, 62 underwent TRA, and 144 underwent TVA. Baseline demographics showed a well-matched distribution. TRA exhibited longer cannulation times but similar procedural and fluoroscopy times. Technical success rates were high for both TRA (98.4%) and TVA (97.2%). Clinical success rates were comparable (96.8% vs. 95.8%). Postprocedure access flow rates and complications demonstrated no significant differences.
Conclusions
This study provides the first direct comparison of TRA and TVA in malfunctioning dialytic fistulas. While venous outflow remains the standard vascular access site for managing malfunctioning dialysis fistulas, TRA shows comparable efficacy, safety, and feasibility, making it a viable alternative in specific clinical contexts. Further studies are needed to confirm these findings and to determine the long-term durability of TRA.
Abbreviations
-
- AVF
-
- Arteriovenous fistula
-
- BB
-
- brachiobasilic
-
- BC
-
- brachiocephalic
-
- CI
-
- confidence interval
-
- cTRA
-
- conventional transradial access
-
- DAP
-
- dose-area product
-
- dRA
-
- distal radial artery
-
- DSA
-
- digital subtraction angiography
-
- dTRA
-
- distal transradial access
-
- KDOQI
-
- Kidney Dialysis Outcomes Quality Initiative
-
- NKF
-
- National Kidney Foundation
-
- PCI
-
- percutaneous coronary intervention
-
- RAO
-
- radial artery occlusion
-
- RAS
-
- radial artery spasm
-
- RC
-
- radiocephalic
-
- TRA
-
- transradial access
-
- TVA
-
- transvenous access
-
- US
-
- ultrasound
-
- VASCs
-
- vascular access site complications
1 Introduction
National Kidney Foundation (NKF) Kidney Dialysis Outcomes Quality Initiative (KDOQI) Clinical Practice Guidelines recommend Arteriovenous fistulas (AVFs) for establishing dialysis access in patients with end-stage renal failure [1]. Stenosis, a common threat to dialysis access patency, can lead to the dysfunction of mature AVFs. Conventionally, percutaneous procedures typically entail either making a venous puncture of the fistula for lesion access and subsequent treatment with balloon angioplasty, in managing stenosis. However, the effectiveness of this approach diminishes when dealing with lesions positioned distal to the puncture site, multiple concurrent lesions involving also the venous outflow, and multiple side branches between the anastomosis and venous access with poorly depiction of the afferent radial artery despite tourniquet application above the venous access site [2-4].
Hence, while venous outflow is the favored choice for vascular access, scenarios exist where arterial access becomes valuable [3, 4]. The arterial access can be established either proximal or distal to the anastomosis site. In particular, adopting a downstream transradial access (TRA) approach offers several advantages. It enables the concurrent treatment of multiple stenoses, some of which might also affect the venous outflow. Additionally, it provides a means to address juxta-anastomotic lesions, especially when there's complex venous anatomy involved. It also simplifies the performance of comprehensive angiography, providing a clear visualization of the entire AVF tract. Moreover, it helps safeguard the arterial inflow against potential complications originating from the vascular access site as opposed to brachial artery access [2, 5, 6].
In recent years, the utilization of TRA in endovascular procedures has garnered significant attention due to its array of benefits compared to traditional transfemoral access. These advantages encompass rapid recovery, a reduced incidence of severe hemorrhagic events, diminished vascular complications, lower rates of adverse cardiovascular events and mortality, ease of hemostasis, and a higher level of patient satisfaction [7-11]. TRA has proven to be effective for the endovascular management of dialytic fistulas as well, since its first description by Wang et al. for interventions on Brescia-Cimino fistulas [2-4].
However, despite these recognized advantages, there remains a paucity of research examining the comparative safety and efficacy of TRA in contrast to venous outflow access for the endovascular management of malfunctioning AVFs. This study is designed to evaluate the effectiveness, feasibility and safety of radial access for the endovascular management of AVFs. Furthermore, it aims to directly compare the efficacy, feasibility and safety data associated with TRA and venous outflow access, addressing the current dearth of comprehensive information in the existing body of literature.
2 Methods
2.1 Study Design
This study is a multi-center (Mater-Domini center of the Dulbecco University Hospital, Catanzaro, Italy; Circolo Hospital, Varese, Italy; Maggiore della Carità University Hospital, Novara, Italy; San Timoteo Hospital, Termoli, Italy) analysis of prospectively collected data of consecutive patients who had undergone, from January 2021 to November 2023, endovascular management of malfunctioning dialytic fistulas with TRA. Inclusion criteria were (I) endovascular treatment for ineffective dialysis due to anastomotic, juxta-anastomotic, venous and/or arterial inflow strictures; (II) radial artery as vascular access site; (III) age between 18 and 85 years; (IV) no prior endovascular procedures for malfunctioning AVF or vascular entries made in arteries of the same upper limb; (V) patency of the radiopalmar arch assessed by the Barbeau Test [12]; VI) evaluation by a multidisciplinary team of nephrologists, vascular surgeons and interventional radiologists. The exclusion criteria were: (I) nonpalpable radial artery at the wrist or radial artery diameter less than 2 mm; (II) completely thrombosed AVF; (III) platelet count < 50,000/μL and/or international normalized ratio > 1.5; (IV) endovascular treatment of central venous stenosis or occlusion; (V) failed-to-mature fistula; (VI) infected fistula; (VII) impending rupture of fistula-related aneurysm.
Patient allocation into the two groups (transradial access and transvenous access) was consistently determined based on preferences expressed by the interventional radiologist and subsequently endorsed during multidisciplinary discussions. Notably, in our institutions, the primary choice of access is often transvenous, with TRA being reserved for specific clinical scenarios, such as multiple stenoses including those affecting venous outflow, stenoses with complex venous anatomy, and juxta-anastomotic strictures challenging to address through prior transvenous access (TVA). In cases where the radial artery was designated as the access point, the choice of distal or proximal conventional TRA was left to the discretion of the operators, provided that the radial access site had a diameter of at least 2 mm. Patients undergoing endovascular management with a venous access site during the same study interval were retrospectively evaluated to constitute a control group. The same indications and treatment technique as in the radial access group were applied to the control group, except for access site hemostasis. Due to the study's retrospective design, ethical committee approval was not deemed necessary. The research followed the ethical guidelines set forth in the Declaration of Helsinki. Before commencing the endovascular procedure, written informed consent was secured from every individual participant.
2.2 Treatment
A comprehensive arterial and venous color Doppler study of the entire limb housing the dialytic fistula was performed no more than 7 days before each intervention. The Barbeau test was performed to assess the patency of the radiopalmar arch. The radial artery was punctured in the conventional location (a few centimeters proximal to the styloid process) or in the distal location (at the anatomical snuffbox). After skin disinfection and local anesthesia, radial artery was punctured under ultrasound guidance and a 4Fr or 5Fr hydrophilic introducer sheath (Glidesheath Slender; Terumo Corp, Tokyo, Japan) was positioned. A spasmolytic cocktail was then administered (200 mcg of Nitroglycerin, 2.5 mg of Verapamil, 2500 IU of unfractionated heparin), to prevent radial artery spasm and occlusion [13]. The procedure was conducted by seasoned consultant-grade interventional radiologists proficient in the endovascular management of dysfunctional dialytic fistulas, employing both TVA and TRA methods. Using a hydrophilic guide wire (Radifocus Guide Wire M Standard Type 0.035” Angled; Terumo Corp, Tokyo, Japan) and a hydrophilic diagnostic catheter (Radifocus Glidecath; Terumo Corp, Tokyo, Japan), the arterial inflow was catheterized a few centimeters above the fistula site, thus performing a comprehensive diagnostic angiography of the fistula, encompassing both the inflow and outflow segments. Venous access was achieved through an ultrasound-guided puncture of the venous outflow, followed by angiographies before and after applying a tourniquet to occlude the venous outflow proximal to the introducer site. Subsequently, any target stenoses were addressed through fistuloplasty, as indicated (e.g., standard high pressure balloons were used according to KDOQI Clinical Practice Guideline) [1]. Finally, a completion angiography was performed from the arterial inflow (Figure 1). Patent hemostasis at the access site was achieved using a TR Band (TR Band; Terumo Corp, Tokyo, Japan) for conventional TRA or compressive bandaging for distal transradial or TVA [14]. The TR Band was removed approximately 4 h later, following confirmation of radial access site hemostasis. We conducted an evaluation of vascular access site complications (VASCs) upon the patient's discharge and again 4 weeks following each treatment. This assessment involved both clinical examination and the use of Doppler ultrasound.
2.3 Outcomes and Definitions
The primary efficacy endpoint is the technical success rate. The secondary efficacy endpoint is the clinical success rate. The primary safety endpoint is defined by the VASC rate. The primary feasibility endpoint is the procedure time.
The “Radial group” includes all patients who underwent TRA, while the “Venous group” includes all patients in whom the vascular access site was the venous outflow. A dialytic fistula was characterized as malfunctioning based on clinical criteria, including recurrent needle clotting, challenging needle insertion, prolonged post-needle removal bleeding times, limb swelling, diminished access flow (less than 500 ml/min), pronounced recirculation (exceeding 15%), decreased blood flow rates, elevated venous pressure, and various other indicators of diminished dialysis effectiveness as assessed by a nephrologist, as well as stenoses of at least 50% as assessed by a sonographer. AVF failure is defined as persistent fistula dysfunction, thus requiring a referral for surgical revisions/creation of a new fistula and/or central venous catheter placement. AVFs were classified according to their anatomical location, namely the radiocephalic fistula, the brachiocephalic fistula, the brachial artery–to–transposed basilic vein (i.e., brachiobasilic fistula) and the others less common [15]. The lesion localization pattern resembles that described by Clark et al. and Shamimi-Noori et al. [16, 17]. Lesions can be categorized into stenoses affecting the inflow arterial segment, those situated at the arteriovenous anastomosis, those in close proximity (within 2 cm) to the anastomosis (namely, “juxta-anastomotic”), and stenoses affecting the venous outflow segment (located at least 2 cm away from the anastomosis). When dealing with multiple stenoses, the most severe stenosis was selected for location classification. Primary patency, assisted primary patency and secondary patency were assessed as in Huijbregts et al. [18]. Secondary patency (access survival until abandonment) was defined as the interval from time of access placement to access abandonment/thrombosis or time of measurement of patency, including intervening manipulations (surgical or endovascular interventions) designed to reestablish the functionality of thrombosed access [18]. Distal radial access was the vascular access performed at the distal part of the radial artery, located at the anatomical snuffbox as described by Kiemeneij [19]. Vascular access site conversion, summarized as “conversion rate”, was the cross-over to another vascular access site to complete the endovascular treatment [20]. Sheath upgrading defines the need of sheath size upgrade to complete the endovascular treatment (e.g., upgrade from 4–5Fr to 6 Fr to perform angioplasty with a larger diameter catheter). Radial artery spasm (RAS) and radial artery occlusion (RAO) were identified through angiography and Doppler ultrasound, respectively. Major bleeding was defined as a hemoglobin drop exceeding 3 g/dL [21, 22]. Technical success is defined as a residual stenosis of less than 30%. Clinical success is defined as the patient's ability to return to effective dialysis using double-needle technique. Procedure-related complications were classified according to the CIRSE Classification System for Complications [23]. Complications were considered clinically significant if they fell within Grade 4 or higher according to the CIRSE classification [23]. Hence, a complication was deemed clinically significant if it resulted in at least mild sequelae. Unless otherwise stated, definitions followed the reporting standards of the Society for Vascular Surgery [24], the KDOQI Clinical Practice Guideline for Vascular Access [1] and other previous investigations [20, 25].
2.4 Statistical Analysis
Data were maintained in an Excel spreadsheet (Microsoft Inc, Redmond, Wash) and the statistical analyses were performed using SPSS software (SPSS, version 22 for Windows; SPSS Inc, Chicago IL, USA). Our analysis focused on the Modified Intention-To-Treat population, encompassing all randomized patients who had received at least one endovascular intervention [26, 27]. Kolmogorov-Smirnov test and Shapiro-Wilk test were used to verify the normality assumption of data [28]. Categorical data are presented as frequency (percentage value) [29]. Continuous normally distributed data are presented as mean ± standard deviation. Continuous not normally distributed data are presented as median (interquartile range: 25th and 75th percentiles—IQR) [30, 31]. The unpaired Student t-test, the Chi-squared/Fisher's exact test, and the Mann-Whitney test were respectively used to assess statistical differences for continuous normally distributed, categorical and continuous not normally distributed data, as appropriate and previously described [32, 33]. Patients’ data were censored at the conclusion of the follow-up period, which extended until November 30, 2023, or a duration of 12 months after the intervention, or at the point of study discontinuation, or when a malfunctioning fistula was definitively abandoned, or in the unfortunate event of a patient's demise. Kaplan-Meier survival analysis was used to evaluate time-dependent outcomes, and the log-rank test was employed to make comparisons. To ensure the independence of censored data from the events being tested, clinical assessments and telephone contacts were conducted in cases of study withdrawal. As a result, the assumption of independent censoring was met, mitigating any potential bias associated with the observed time-dependent data. A P-value of < 0.05 was considered statistically significant for the abovementioned tests.
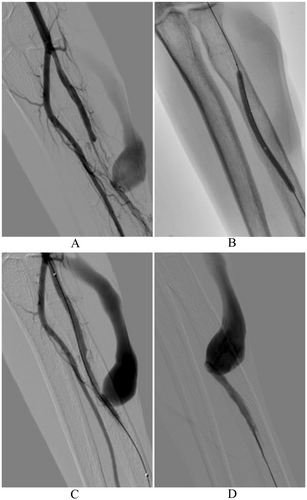
3 Results
3.1 Study Population
A total of 206 patients participated in the study, with 62 undergoing transradial access (TRA) and 144 undergoing transvenous access (TVA). The mean age for all patients was 66.5 years (±11.8). There was no statistically significant difference in age between the TRA group (mean = 68 years, ±1.4) and the TVA group (mean = 65.9 years, ±1.3) (p = 0.197). Gender distribution showed 62.6% male and 37.4% female, and while there was a trend toward more males in the TVA group (p = 0.377), the difference was not statistically significant. The prevalence of comorbidities such as hypertension, cerebrovascular disease, coronary artery disease, smoking history, current smoking, diabetes, hyperlipidemia, and coagulopathy demonstrated no significant differences between TRA and TVA groups, with corresponding p-values provided. INR, aPTT, platelet count, and the use of antiplatelet and anticoagulant therapy also exhibited comparable values between the two access types, as denoted by their respective p-values. In summary, the Table 1 offers a detailed overview of patient characteristics. Baseline demographic data indicate a well-matched distribution between the TRA and TVA groups across various demographic and clinical parameters.
| Variables | All Patients (n = 206) | |||
|---|---|---|---|---|
| Transradial access (n = 62) | Transvenous access (n = 144) | p-value | ||
| Age (years) | 66.5 (±11.8) | 68 (±1.4) | 65.9 (±1.3) | 0.197 |
| Sex (M/F) | 129 (62.6%)/77 (37.4%) | 37 (59.7%)/25 (40.3%) | 92 (63.9%)/52 (36.1%) | 0.377 |
| Hypertension | 116 (56.3%) | 34 (54.8%) | 82 (56.9%) | 0.449 |
| Cerebrovascular disease | 61 (29.6%) | 19 (30.6%) | 42 (29.2%) | 0.478 |
| Coronary artery disease | 79 (38.3%) | 23 (37.1%) | 56 (38.9%) | 0.468 |
| Smoking history | 144 (69.9%) | 44 (71%) | 100 (69.4%) | 0.482 |
| Current smoker | 90 (43.7%) | 30 (48.3%) | 60 (41.7%) | 0.230 |
| Diabetes | 98 (47.6%) | 32 (51.6%) | 66 (45.8%) | 0.271 |
| Hyperlipidemia | 126 (61.2%) | 40 (64.5%) | 86 (59.7%) | 0.313 |
| INR | 1.35 (±0.3) | 1.36 (±0.3) | 1.34 (±0.3) | 0.721 |
| aPTT (s) | 39.2 (±5.7) | 39.5 (±5.4) | 39.1 (±5.9) | 0.584 |
| Platelet count (No. ×103/μL) | 333.7 (±127.8) | 347.3 (±125.2) | 327.8 (±128.9) | 0.371 |
| Coagulopathy | 86 (41.7%) | 28 (45.2%) | 58 (48.3%) | 0.308 |
| Antiplatelet therapy | 102 (49.5%) | 28 (45.2%) | 74 (51.4%) | 0.252 |
| Anticoagulant therapy | 98 (47.6%) | 30 (48.4%) | 68 (47.2%) | 0.499 |
- Abbreviations: μL, microliter; aPTT, activated partial thromboplastin time; F, female; INR, International Normalized Ratio; M, male; s, seconds.
3.2 Procedure Data
Table 2 compares procedural data between transradial access (TRA) and transvenous access (TVA) groups in the endovascular management of malfunctioning dialytic fistulas. TRA exhibited a distinct distribution of fistula types, with higher percentages of radiocephalic (11.3% vs. 8.1%) and brachiobasilic (33.9% vs. 22.2%) fistulas compared to TVA (p = 0.003). While stenosis characteristics did not significantly differ, the TRA group showed longer cannulation time (103.4 s vs. 86.5 s, p < 0.001) and lower contrast volume (42.6 mL vs. 54 mL, p < 0.001). Successful cannulation and sheath introduction were 100% for both groups. While there was a trend toward more access site punctures in the TRA group, the difference was not statistically significant (p = 0.058). Despite longer cannulation times, TRA group showed similar procedural (48.2 min vs. 45.6 min, p = 0.357) and fluoroscopy (10 min vs. 9.7 min, p = 0.410) times to TVA group. Radiation exposure metrics were not significantly different between the two groups.
| Variables | All Patients (n = 206) | |||
|---|---|---|---|---|
| Transradial access (n = 62) | Transvenous access (n = 144) | p-value | ||
| Fistula | ||||
|
55 (26.7%) | 7 (11.3%) | 48 (33.3%) | |
|
67 (32.5%) | 27 (43.5%) | 40 (27.8%) | 0.003 |
|
53 (25.8%) | 21 (33.9%) | 32 (22.2%) | |
|
31 (15%) | 7 (11.3%) | 24 (16.7%) | |
| Side (Right/Left) | 78 (37.9%)/128 (62.1%) | 28 (45.2%)/34 (54.8%) | 50 (34.7%)/94 (65.3%) | 0.163 |
| Pre-procedure access flow rate (mL/min) | 556.1 (±262.5) | 504 (±36.7) | 578.5 (±310.7) | 0.418 |
| Stenosis length | ||||
|
78 (37.9%) | 18 (29%) | 60 (41.7%) | |
|
80 (38.8%) | 26 (41.9%) | 54 (37.5%) | 0.192 |
|
48 (23.3%) | 18 (29%) | 30 (20.8%) | |
| Stenosis location | ||||
|
9 (4.4%) | 5 (8.1%) | 4 (2.8%) | |
|
55 (26.7%) | 15 (24.2%) | 40 (27.8%) | 0.281 |
|
66 (32%) | 22 (35.5%) | 44 (30.6%) | |
|
76 (36.9%) | 20 (32.2%) | 56 (38.8%) | |
| Number of access site punctures | 1.2 (±0.5) | 1.3 (±0.6) | 1.1 (±0.4) | 0.058 |
| Successful cannulation and sheath introduction | 100 (100%) | 100 (100%) | 100 (100%) | NA |
| Cannulation time (s) | 91.6 (±33.2) | 103.4 (±25.5) | 86.5 (±34.9) | < 0.001 |
| Introducer sheath size | ||||
|
25 (12.1%) | 11 (17.7%) | 14 (9.7%) | |
|
152 (73.8%) | 50 (80.6%) | 102 (70.8%) | 0.004 |
|
22 (10.7%) | 0 (0%) | 22 (15.3%) | |
|
7 (3.4%) | 1 (1.6%) | 6 (4.2%) | |
| Introducer sheath upgrade | 8 (3.9%) | 2 (3.2%) | 6 (4.2%) | 0.549 |
| Vascular access site conversion | 3 (1.5%) | 1 (1.6%) | 2 (1.4%) | 0.661 |
| Contrast volume (mL) | 50.6 (±16.1) | 42.6 (±12.3) | 54 (±16.4) | < 0.001 |
| Procedure duration (min) | 46.4 (±14) | 48.2 (±13.3) | 45.6 (±14.2) | 0.357 |
| Fluoroscopy time (min) | 9.8 (±3.5) | 10 (±3) | 9.7 (±3.7) | 0.410 |
| Cumulative air kerma (mGy) | 190.5 (±65) | 194.2 (±57.9) | 188.9 (±68) | 0.652 |
| Dose area product (DAP) (Gy/cm2) | 22.4 (±8.5) | 22.2 (±7.6) | 22.4 (±8.9) | 0.916 |
- Abbreviations: cm, centimeters; Gy, gray; min, minutes; mL, milliliter; s, seconds.
3.3 Efficacy and Safety Outcomes
Technical success rates were high and comparable between TRA (98.4%) and TVA (97.2%) groups, with no statistically significant difference (p = 0.525). Similarly, clinical success rates demonstrated no significant variance, standing at 96.8% for TRA and 95.8% for TVA (p = 0.549). Postprocedure access flow rates showed no significant difference between TRA (987.5 mL/min) and TVA (1055.7 mL/min) groups, with an average increase in access flow rate of 483.5 mL/min for TRA and 477.2 mL/min for TVA. The overall procedure-related complication rate was 9.7%, evenly distributed between TRA and TVA (p = 1), with vascular access site complication rates at 6.5% for TRA and 5.6% for TVA (p = 0.513). Post-angioplasty venous rupture was noted in six instances (4.2%) in the TVA group and in two instances (3.2%) in the TRA group; all cases were effectively managed with manual compression. Most complications were minor (CIRSE classification grades 1–3), requiring medical or percutaneous intervention, while none reached major classification (grades 4–6). Notably, all complications were effectively managed, with no required surgical intervention. Details are given in Table 3.
| Variables | All Patients (n = 206) | |||
|---|---|---|---|---|
| Transradial access (n = 62) | Transvenous access (n = 144) | p-value | ||
| Technical success | 201 (97.6%) | 61 (98.4%) | 140 (97.2%) | 0.525 |
| Clinical success | 198 (96.1%) | 60 (96.8%) | 138 (95.8%) | 0.549 |
| Postprocedure access flow rate (mL/min) | 1035.2 (±250.7) | 987.5 (±81.5) | 1055.7 (±293) | 0.612 |
| Average increase in access flow rate (mL/min) | 479.1 (±126.6) | 483.5 (±72.7) | 477.2 (±143.9) | 0.367 |
| Procedure-related complication rate | 20 (9.7%) | 6 (9.7%) | 14 (9.7%) | 1 |
| Vascular access site complication rate | 12 (5.8%) | 4 (6.5%) | 8 (5.6%) | 0.513 |
| Vascular access site complication | ||||
|
194 (94.2%) | 58 (93.5%) | 136 (94.4%) | |
|
10 (4.8%) | 2 (3.2%) | 8 (5.6%) | 0.162 |
|
1 (0.5%) | 1 (1.6%) | 0 (0%) | |
|
1 (0.5%) | 1 (1.6%) | 0 (0%) | |
| Procedure-related complications (CIRSE classification) | 186 (90.3%) | 56 (90.3%) | 130 (90.3%) | |
|
20 (9.7%) | 6 (9.7%) | 14 (9.7%) | |
|
0 (0%) | 0 (0%) | 0 (0%) | 1 |
|
||||
| Required treatment for complications | ||||
|
0 (0%) | 0 (0%) | 0 (0%) | |
|
20 (100%) | 6 (100%) | 14 (100%) | NA |
|
0 (0%) | 0 (0%) | 0 (0%) | |
|
0 (0%) | 0 (0%) | 0 (0%) | |
- Abbreviations: min, minutes; mL, milliliter.
3.4 Survival Analysis
By Kaplan-Meier survival analysis, primary patency rates were 95.2% (±0.03 SE) at 1 month, 75.8% (±0.05 SE) at 6 months and 48.4% (±0.06 SE) at 12 months in TRA group. Primary patency rates were 94.4% (±0.02 SE) at 1 month, 77.8% (±0.03 SE) at 6 months and 41.7% (±0.04 SE) at 12 months in TVA group. Assisted-primary patency rates were 95.2% (±0.03 SE) at 1 month, 88.7% (±0.04 SE) at 6 months and 48.4% (±0.06 SE) at 12 months in TRA group. Assisted-primary patency rates were 94.4% (±0.02 SE) at 1 month, 86.1% (±0.03 SE) at 6 months and 41.7% (±0.04 SE) at 12 months in TVA group. Secondary patency rates were 95.2% (±0.03 SE) at 1 month, 88.7% (±0.04 SE) at 6 months and 61.1% (±0.06 SE) at 12 months in TRA group. Secondary patency rates were 94.4% (±0.02 SE) at 1 month, 87.5% (±0.03 SE) at 6 months and 62.4% (±0.04 SE) at 12 months in TVA group. The median primary patency wasn't significantly different between TRA group (12 months, CI: NA-NA) and TVA group (11 months, CI: 9.6-12.4), according to the Log-Rank test (p = 0.469). For patients undergoing TRA, the median (CI) assisted-primary patency was 12 (NA-NA) months, similar to that of patients undergoing TVA (12 [11.5–12.5] months) (p = 0.477, calculated by mean of Log-Rank test). The median secondary patency wasn't significantly different between TRA group (12 months, CI: NA-NA) and TVA group (12 months, CI: NA-NA), according to the Log-Rank test (p = 0.863) Table 4.
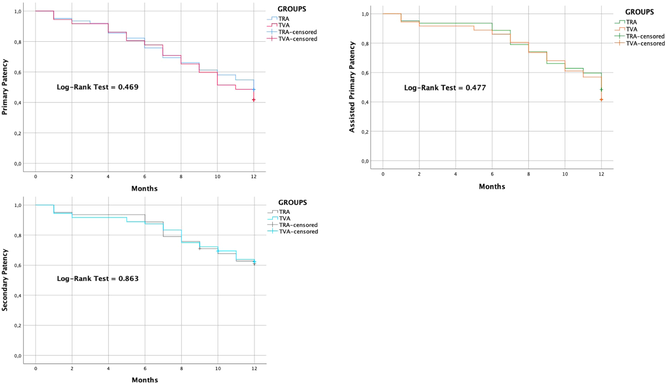 .
.| Cumulative Rates | At 1 month rate (±SE)—numbers at risk |
At 6 months rate (±SE)—numbers at risk |
At 12 months rate (±SE)—numbers at risk |
|
|---|---|---|---|---|
 |
Primary patency TRA |
95.2% (±0.03) - 59 | 75.8% (±0.05) - 47 | 48.4% (±0.06) - 30 |
 |
Primary patency TVA |
94.4% (±0.02) - 136 | 77.8% (±0.03) - 112 | 41.7% (±0.04) - 60 |
 |
Assisted-primary patency TRA |
95.2% (±0.03) - 59 | 88.7% (±0.04) - 55 | 48.4% (±0.06) - 30 |
 |
Assisted-primary patency TVA | 94.4% (±0.02) - 136 | 86.1% (±0.03) - 124 | 41.7% (±0.04) - 60 |
 |
Secondary patency TRA |
95.2% (±0.03) - 59 | 88.7% (±0.04) - 55 | 61.1% (±0.06) - 37 |
 |
Secondary patency TVA |
94.4% (±0.02) - 136 | 87.5% (±0.03) - 126 | 62.4% (±0.04) - 88 |
4 Discussion
-
Transradial access demonstrates comparable efficacy (technical and clinical success rates) to transvenous access for the endovascular management of malfunctioning dialytic fistulas. This holds true despite its application in clinically challenging scenarios, including multiple stenoses involving venous outflow, complex venous anatomy, and difficult-to-cross juxta-anastomotic stenoses that were previously addressed via transvenous access.
-
Transradial access is proven to be safe, with no significantly higher rates of vascular access site complications when compared to transvenous access. It is essential to note that this finding is specific to the clinical conditions of our study. Notably, the assessment of radiopalmar arch patency and the considerable experience of operators in performing transradial access may have played a crucial role in mitigating radial vascular access site complications.
-
The feasibility of transradial access is excellent, as the procedural time is similar to that of transvenous access, and there were minimal instances of intraprocedural conversion to alternative venous access or upgrading to introducers of at least 6 French.
TRA is widely employed in the field of endovascular interventions, including the management of dysfunctional dialytic fistulas [2, 4, 19, 34, 35]. Rahmatzadeh et al. observed a 100% technical success rate in 30 interventions performed via TRA for endovascular management of dysfunctional dialytic AVF. A technical success rate of 95.3% was noted in an investigation by Ong et al. on a large cohort of patients from Singapore, despite smaller radial artery diameters than Western population [2]. Wang et al. enrolled 50 dysfunctional Brescia-Cimino fistulas and 69 target lesions in their study, with only a case of TRA technical failure due to intense RAS after failed radial artery puncture and five cases of residual stenosis greater than 30% (namely, 91.3% technical success rate) [3]. Technical and clinical success rates of 98.6% and 91.7%, respectively, were noted by Choi et al. following angioplasty of 73 malfunctioning AVFs [36]. The NKF KDOQI guidelines specify a minimum access flow rate of 500 mL/min to ensure effective dialysis [1]. In our study, all patients exhibited a significant improvement in access flow rate, ensuring effective dialysis (namely, 100% clinical success rate). These results are consistent with a few other reports on TRA, such as those by Mohiuddin et al. and Ong et al. [2, 37]. Kawarada et al. reported 100% technical and clinical success rates in their case-series on 50 radiocephalic fistulas treated by TRA [4].
While it is common practice to consider venous outflow as the primary vascular access site [38] and to use TRA in specific clinical scenarios for the endovascular management of malfunctioning dialytic fistulas [5], there is a notable lack of studies that have directly compared TVA and TRA. The efficacy of TRA procedures may be constrained by the presence of narrow radial artery diameters, thus preventing the use of larger caliber catheters [39]. Our study's results indicate that both TVA and TRA routes are associated with high rates of technical and clinical success. Furthermore, the direct comparison did not reveal statistically significant differences between the two groups, thus making TRA equally effective to TVA for endovascular management of malfunctioning dialytic fistulas. Hence, TRA access should be considered in specific challenging clinical scenarios. Firstly, in the presence of multiple stenoses that also affect the venous outflow, as selecting an appropriate transvenous access site can be complex in such instances, potentially necessitating a distant puncture near the axilla. Secondly, in cases involving complex venous anatomy and stenoses, TRA allows for the effortless execution of arterial inflow angiography, ensuring a clear visualization of the entire fistula conduit and enhanced maneuverability of the guide within the intricate network of veins (e.g., as seen in the Gracz fistula where the perforating vein of the median antebrachial vein is anastomosed with the proximal radial artery) [40, 41]. Thirdly, in situations where the transvenous approach fails to traverse tight stenoses (e.g., 65% technical success rate reported for peripheral occlusion of radiocephalic fistulas addressed with TVA) [3, 42]. Lastly, it is important to take into account that applying hemostasis directly to the venous outflow fistula can increase the risk of fistula thrombosis, particularly when the outcomes of percutaneous transluminal angioplasty (PTA) are suboptimal [3].
Another viable approach is the transbrachial access, which has been evaluated in comparison with the TRA by Shamimi-Noori et al. In a cohort of 56 patients undergoing endovascular interventions for nonmaturing AVFs, the subgroup subjected to TRA exhibited fewer access site punctures, higher rates of clinical and technical success, superior primary patency, and improved assisted primary patency at 12 months [17].
While the assessment of short- and medium-term patency is not the primary focus of this study, the recorded data align with findings from other research within the realm of endovascular management of AVFs. Rahmatzadeh et al. recorded a 32.8% primary patency rate and a 63.3% assisted primary patency rate at 12 months, in a cohort of 30 patients undergoing endovascular management of malfunctioning fistulas with TRA [5]. Ong et al. observed a poor primary patency rate of 19.5% at 12 months in patients from Singapore [2]. Evaluations of PTA for dialysis access interventions involving the customary TVA method indicate a spectrum of primary patency rates at the 6-month mark, spanning from 50% to 70%, as documented in existing studies [43].
In the case series on TRA by Rahmatzadeh et al., 46.7% patients required an upgrade to a 6-Fr sheath for the final intervention [5]. In our study, the rate of upgrading to an introducer with a minimum diameter of six French was relatively low compared to earlier studies. Several factors may contribute to this trend, including the limited number of cases involving multiple stenoses including central venous ones, the exclusion of isolated central venous stenoses and the technological advancements that have allowed the use of low-profile larger caliber PTA balloons compatible with five French introducers (e.g., Sterling balloon catheter, Boston Scientific Corporation, Natick, MA). Moreover, the feasibility of TRA is underscored by the absence of significant differences in procedural time between the TRA and TVA groups in our experience. Consequently, our findings suggest that TRA did not entail a significant procedural time extension compared to TVA. This observation might be explained by the fact that, despite TRA being associated with a longer cannulation time, the ease of performing an angiography from the arterial inflow potentially facilitates quicker visualization and swifter crossing of target stenoses. Lastly, our findings align with other reports on fistuloplasty using TRA, which have documented average procedural times of approximately 30–50 min [2, 3, 44, 45].
In our investigation, all VASCs remained clinically inconsequential and were managed with noninvasive therapies. Specifically, cases of RAS and RAO were pharmacologically treated, while effective resolution of access site hematomas and post-fistuloplasty venous ruptures was achieved through prolonged compression. The rate of vascular access site complications (VASCs) was comparable to other published studies in the endovascular field, whether it is femoral or radial access [46-56]. Shamini-Noori et al. also found a significantly lower rate of complications in the TRA group compared to the transbrachial access group for percutaneous management of nonmaturing AVFs [17]. The occlusion of the radial artery represents one of the most concerning complications associated with TRA, with its incidence ranging from 1% to 10% [57]. This risk has been further mitigated by the growing adoption of distal radial access in clinical practice [20, 47, 58, 59]. The incidence of clinically significant RAO is quite rare, primarily owing to the anastomoses between the ulnar and radial arteries [57, 60]. Rahmatzadeh et al. described two cases (6.7%) of RAO with good supply to the palmar arch by patent ulnar arteries; no clinically-significant VASCs were noted [5]. In a recent investigation on 43 fistuloplasties with TRA by Ong et al., a 9.3% rate of RAO was noted and all instances were managed conservatively [2]. Kawarada et al. didn't observe any cases of RAO in their series on 50 radiocephalic fistulas treated by TRA [4]. In our case series, only one instance of radial artery occlusion has been recorded, which remained clinically inconsequential thanks to the robust ulnar artery supply, a factor consistently assessed through the Barbeau Test before every TRA procedure [12]. Additionally, it can be speculated that the low rate of RAO observed in our study is influenced by the ultrasound assessment of vascular caliber adequacy before each TRA [61], the US-guided puncture [5], the substantial expertise of operators in performing TRA procedures [62], the use of thin-walled sheaths [63] as well as catheters and guides with hydrophilic coating [64], the administration of spasmolytic cocktail after introducer sheath positioning [13, 65], the adoption of a patent hemostasis technique [66], and the reduced application time of the hemostatic device [67]. Therefore, TRA was not associated with a significantly higher rate of VASCs in comparison to TVA, and no clinically significant VASCs were noted, in keeping with previous evidence. The use of TRA appears to be a safe option for the endovascular management of malfunctioning dialytic fistulas.
It is essential to underscore certain anatomical limitations of the TRA approach. A minority of patients may have a radial artery caliber measuring less than 2 mm, making the use of the radial access site inadvisable [61]. Another anatomical constraint associated with TRA is its inability to accommodate larger caliber introducers. The need to upgrade to larger caliber introducers may arise in the presence of central venous stenoses, thromboses addressed with thrombectomy device or when deploying covered stents for vascular ruptures. In certain instances, the radial artery's diameter permits the placement of eight French introducers, albeit at the cost of an elevated risk of vascular injury and RAO [68, 69]. The inability to navigate across the arteriovenous anastomosis from a TRA can occur due to variant anatomical factors, such as a radial loop, acute angulation at the anastomosis, a high origin of the radial artery, or stenosis [17, 20, 48]. The TRA may have limitations when dealing with arterio-venous anastomoses that are extremely distal and positioned close to the radial access site. In such situations, it can be beneficial to explore the option of a distal TRA, typically within the anatomical snuffbox region [17, 70]. Interestingly, an atypical origin of the radial artery was previously noted in 8.3% patients undergoing TRA [71].
The retrospectivity of the analysis and the non-randomized fashion are the main limitations of our study. Another notable limitation is the extensive experience of our operators in TRA procedures. It is well-established that TRA necessitates a learning curve, and vascular access site complications are less frequent in centers with greater TRA utilization [62]. Thus, it is plausible that the low VASC rate observed may, in part, be influenced by operator expertise. Consequently, our findings may not be readily generalizable to centers with limited TRA experience. Another limitation stems from the substantial influence of methodological aspects on the safety of TRA. Indeed, the meticulous attention to numerous procedural details may have significantly contributed to the reduction of VASCs, thus rendering the findings of our study less applicable to clinical scenarios lacking this level of methodological rigor. The criteria for selecting TRA differed from those for TVA, potentially introducing a selection bias. Patient allocation was not randomized but rather based on operator preferences endorsed in multidisciplinary discussions. Addressing this bias analytically could be achieved through propensity score matching, which requires treatment group assignment to depend on observable variables. However, in our case, assignment is contingent upon operator preferences, which are not observable variables and cannot be quantified as covariates. Nevertheless, we do not believe this has impacted the outcomes of our study, given that our operators employ TRA in specific clinical scenarios, which are inherently more challenging than the typical TVA. Consequently, the effectiveness associated with TRA should be diminished rather than enhanced by these criteria. The measurement of secondary patency did not differentiate the reasons for AVF abandonment; thus, it was not recorded how many AVFs were specifically abandoned due to thrombosis. However, no cases of outflow thrombosis were recorded as complications related to the performed endovascular procedure. Lastly, an assessment of long-term results was not conducted.
5 Conclusions
To the best of our knowledge, no study has directly compared the effectiveness, feasibility, and safety of TRA with TVA for the endovascular management of malfunctioning dialysis fistulas.
Our report highlights remarkably high rates of both technical and clinical success with TRA, which are akin and comparable to standard TVA. The feasibility of TRA is optimal when considering non-prolonged procedural times and infrequent instances of conversion to another vascular access or upgrading to a six French introducer or larger. TRA stands as a secure vascular approach widely embraced in clinical practice, and it demonstrates no statistically significant increase in clinically relevant VASCs when compared to TVA. While venous outflow remains the standard vascular access site for managing malfunctioning dialysis fistulas, TRA can prove highly effective and safe in certain challenging clinical scenarios.
Acknowledgments
Open access publishing facilitated by Universita degli Studi Magna Graecia di Catanzaro, as part of the Wiley - CRUI-CARE agreement.
Conflicts of Interest
The authors declare no conflicts of interest.
Ethics Statement
Ethics committee approval was not required due to the retrospective nature of the study. The study has been conducted in accordance with the Helsinki Declaration. All patients signed a written informed consent before receiving the endovascular treatment.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.




