Impact of residual mitral regurgitation after transcatheter edge-to-edge repair in atrial functional mitral regurgitation: Results from MITRA-PRO registry
[Correction added on 1 April 2025, after first online publication: Dennis Rottländer has been listed as a co-corresponding author of the article.]
Abstract
Background
Transcatheter edge-to-edge repair (TEER) has emerged to address symptomatic atrial functional mitral regurgitation (aFMR) in patients who are at high operative risk.
Aims
No clinical data is available on the impact of residual mitral regurgitation (MR) following TEER in aFMR compared to ventricular functional MR (vFMR).
Methods
In the MITRA-PRO registry, 846 patients with FMR and MitraScore assessment for residual MR quantification were included (722 patients with vFMR and 124 patients with aFMR).
Results
Compared to vFMR similar procedural results in regard of residual MR following TEER were found in aFMR patients (MitraScore post TEER 2.5 ± 1.8 vs. 2.7 ± 1.9), while the amount of implanted TEER devices was increased in vFMR. 1-year survival was better in aFMR compared to vFMR regardless of relevant residual MR (MitraScore ≥ 4), while 1-year rehospitalization was comparable for both MR entities. Patients with aFMR and mild residual MR had a lower mortality rate (6.6% vs. 10.3%) and rehospitalization rate (29.1% vs. 46.2%) 1 year after mitral TEER. However, in contrast to vFMR a MitraScore ≥4 was no independent predictor of mortality in aFMR indicating a better tolerance toward residual MR.
Conclusions
Residual MR is an independent predictor of 1-year mortality in vFMR patients, whereas in aFMR patients, a MitraScore of ≥4 is associated with higher mortality but is not an independent predictor in multivariate analysis. Therefore, minimizing MR through mitral TEER is crucial for survival in vFMR patients, while aFMR patients tolerate significant residual MR better 1 year after the procedure.
1 INTRODUCTION
Atrial functional mitral regurgitation (aFMR) is associated with left atrial (LA) and mitral annular dilatation most often due to atrial fibrillation (AF) or heart failure with preserved ejection fraction (HFpEF). aFMR was found in up to 53% of HFpEF patients.1 However, the exact prevalence of aFMR in the general population is unknown and seems currently to be underreported.2 Characteristics of aFMR are normal LV-function, normal mitral leaflet motion, a more central mitral regurgitation (MR) jet and severe LA dilatation. This is in contrast to ventricular secondary mitral regurgitation (vFMR), where systolic left ventricular (LV) dysfunction, restricted leaflet motion, an increased tethering force and LA dilatation are pathogenetic. However, severity of MR at discharge after acute decompensation in HFpEF was linked to worse clinical outcome implicating a need for therapeutic options.3 Transcatheter mitral valve repair is an emerging therapeutic option in aFMR, but currently no specific guidelines exist.2 However, mitral transcatheter edge-to-edge repair (TEER) is effective and safe in the treatment of aFMR and improved heart failure symptoms in long-term follow-up.4 Among patients with mitral TEER between 7.5% and 12.8% of the patients showed aFMR in large multicenter registries indicating a relevant patient population.4-7 Recently the prospective large scale multicenter MITRA-PRO registry revealed that residual MR assessed by the MitraScore is associated with 1-year mortality and rehospitalization in patients with functional MR (FMR) undergoing mitral TEER.8 For residual MR quantification the validated and multimodal MitraScore was used, which included both echocardiographic and hemodynamic parameters.8, 9 However, little is known about the impact of residual MR on the prognosis of patients with aFMR especially in comparison to vFMR. One might speculate, that patients with HFpEF might tolerate residual MR after mitral TEER for a longer time compared to patients with vFMR. Therefore, we aimed to evaluate the role of residual MR following TEER in patients with aFMR and compare the results to vFMR.
2 METHODS
2.1 Study population
The results of the MITRA-PRO (MITRAclip PROgnosis) registry were recently published (German Clinical Trials Register: DRKS00012288).8 This prospective, multicenter registry revealed the association of residual intraprocedural MR following TEER and 1-year mortality.8 It was performed according to good clinical practice and in compliance with the Helsinki declaration and an individual written consent was obtained by every patient. The study was approved by the ethical committee of the University of Witten-Herdecke (approval number: 96/2016) or the local ethic committees of the participating centers.
The MITRA-PRO registry included 1546 patients of 24 German centers as previously published.8 All patients were included from August 01, 2016 until April 01, 2020. Intraprocedural MitraScore was assessed in 1491 patients prior and post mitral TEER. Clinical follow-ups were done investigator independent by a central site (ZKS Witten/Herdecke University).
2.2 Mitral TEER
Mitral TEER was performed using either MitraClip XT, NT, NTR or XTR (Abbott Structural Heart) as previously published.10
2.3 Definition of aFMR and vFMR
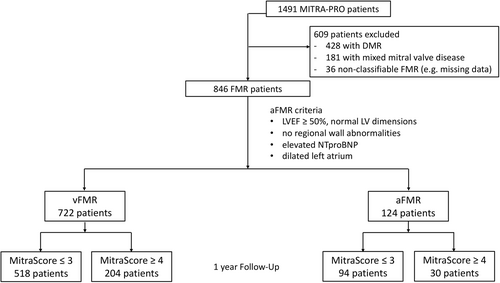
Patients with degenerative MR, mixed mitral valve disease or nonclassifiable FMR (poor imaging quality, missing data) were excluded from this subanalysis of the MITRA-PRO registry. MITRA-PRO FMR patients were classified as aFMR, when (1) LV ejection fraction (LVEF) was ≥50%, (2) no regional wall abnormalities were present, (3) LA was dilated (LA diameter >4.0 cm or LA area >20 cm2 or indexed LA volume >34 mL/m2) (4) NT-proBNP was elevated and (5) LV dimensions were normal (indexed LV end-diastolic volume ≤74 mL/m2 for men and ≤61 mL/m2 for women). Patients with reduced LVEF < 50% were included into the vFMR group.
2.4 MitraScore
In the MITRA-PRO registry, MitraScore was performed to assess residual MR as previously described.8, 9 The multimodal MitraScore was assessed as follows: (1) hemodynamic assessment of LA v-wave (0–3 points); (2) angiographic assessment of MR during LV angiography (0–3 points); (3) two-dimensional echocardiographic assessment of MR severity (0–3 points) including extra points for eccentric MR jet and flow reversal in the pulmonary veins; and (4) echocardiographic assessment of regurgitant bubbles during LV volume challenge similar to the surgical saline testing (0–3 points).9 Therefore, the MitraScore ranges from 0 to 14 points, and a postprocedural MitraScore ≤3 points was defined as no or mild residual MR. A MitraScore ≥4 points was considered as relevant residual MR. Of note, in patients not undergoing a LV angiogram on investigators choice, for example, in patients with chronic renal failure, a modified MitraScore was available. In this case the 6 corresponding points of the LV angiogram were replaced by 3D Vena Contracta Area (3D-VCA).
2.5 Intraprocedural hemodynamics
TEER was performed without exceeding the threshold of 5 mmHg mean pressure gradient across the mitral valve determined by continuous wave Doppler in transesophageal echocardiography. Hemodynamic MR parameters were checked after guide insertion and 10 min after detachment of the last TEER device. Investigators aimed for a LV end-diastolic pressure >10 mmHg or a central venous pressure between 10 and 15 mmHg. Furthermore, systolic arterial blood pressure was adjusted to the patient's preprocedural level by norepinephrine and/or fluid substitution.
2.6 Statistical analysis
Categorical parameters are described using absolute numbers and percentages and were compared using the Pearson chi-square test, continuous variables were presented as mean and standard deviation or median and quartiles and were compared using the Kruskal-Wallis test. The cumulative incidence of mortality and of the combined endpoint (mortality and/or reintervention) were visualized as Kaplan-Meier curves with patients at risk and compared using log-rank test. Centralized follow-up was performed by the Centre for Clinical Studies of the University of Witten/Herdecke at 12 months with a time-window of ±4 weeks. Therefore, follow-ups conducted during the 1-year follow-up suggest a lower rate for patients at risk on day 360 than the 96.6% completeness achieved in the MITRA-PRO registry. Distribution of NYHA and preprocedural MR classes at baseline, discharge and 12-months follow-up was represented as bar chart. All analyzes were performed using SAS release 9.4 (Statistical Analysis Software).
3 RESULTS
The MITRA-PRO registry included 1491 consecutive patients with degenerative, functional or mixed (degenerative-functional) MR undergoing mitral TEER and residual MR quantification using the multimodal MitraScore. After exclusion of patients with degenerative MR, mixed MR or nonclassifiable FMR 846 patients with FMR were identified and either classified as aFMR or vFMR (Figure 1). In this subanalysis 722 patients with vFMR and 124 patients with aFMR were included (Figure 2A).
Baseline characteristics of aFMR and vFMR groups are shown in Table 1. Patients in the aFMR group were predominantly female (69.4%) compared to vFMR (35.5%). Furthermore, mean age was significantly higher in the vFMR group, while logistic Euroscore was lower compared to aFMR (76.0 ± 9.0 vs. 81.0 ± 5.0 years and 25.0 ± 15.7 vs. 16.4% ± 11.1%, p < 0.001). As expected, diabetes mellitus and coronary artery disease were more frequent in vFMR, while arterial hypertension was found in 91.1% in aFMR and 83.4% in vFMR (Table 1). LVEF was normal in aFMR and significantly reduced in vFMR (58.5 ± 5.3 vs. 35.2% ± 12.8%, p < 0.001), while systolic pulmonary artery pressure (sPAP) was comparable in both groups (51.0 ± 29.9 vs. 47.3 ± 14.2 mmHg, p = 0.66). Right heart function was more impaired in vFMR (Table 1). Functional status assessed by NYHA classification and 6-min walking distance was comparable in both groups (Table 1). However, NTproBNP was markedly elevated in vFMR (4246.5 vs. 2107.0, <0.001) due to the reduction in LVEF.
| vFMR | aFMR | ||||
|---|---|---|---|---|---|
| n = 722 | n = 124 | ||||
| n | % or Mean ± SD | n | % or Mean ± SD | p value | |
| Age | 722 | 76.0 ± 9.0 | 124 | 81.0 ± 5.0 | <0.001 |
| Female | 256 | 35.5 | 86 | 69.4 | <0.001 |
| Log EuroScore (%) | 656 | 25.0 ± 15.7 | 117 | 16.4 ± 11.1 | <0.001 |
| Patients' history | |||||
| Arterial hypertension | 602 | 83.4 | 113 | 91.1 | 0.028 |
| Diabetes mellitus | 243 | 33.7 | 21 | 16.9 | <0.001 |
| Coronary artery disease | 497 | 68.8 | 38 | 30.6 | <0.001 |
| Ischemic cardiomyopathy | 327 | 45.3 | 0 | 0.0 | <0.001 |
| Nonischemic cardiomyopathy | 233 | 32.3 | 0 | 0.0 | <0.001 |
| Pacemaker | 171 | 23.7 | 27 | 21.8 | 0.64 |
| ICD | 327 | 45.3 | 0 | 0.0 | <0.001 |
| CRT | 130 | 18.0 | 2 | 1.6 | <0.001 |
| Atrial fibrillation | 479 | 66.3 | 103 | 83.1 | 0.46 |
| Tricuspid regurgitation (≥moderate) | 384 | 53.2 | 65 | 52.5 | 0.88 |
| Echocardiography | |||||
| LVEF (%) | 721 | 35.2 ± 12.8 | 124 | 58.5 ± 5.3 | <0.001 |
| sPAP (mmHg) | 695 | 47.3 ± 14.2 | 117 | 51.0 ± 29.9 | 0.66 |
| MR grading | |||||
| No MR | 0 | 0.0 | 0 | 0.0 | 0.020 |
| MR mild | 1 | 0.1 | 2 | 1.6 | |
| MR moderate | 71 | 9.8 | 19 | 15.3 | |
| MR severe | 650 | 90.0 | 103 | 83.1 | |
| TR grading | |||||
| No TR | 44 | 6.1 | 11 | 8.9 | 0.88 |
| TR mild | 294 | 40.7 | 48 | 38.7 | |
| TR moderate | 269 | 37.3 | 39 | 31.5 | |
| TR severe | 115 | 15.9 | 26 | 21.0 | |
| Right heart function | |||||
| RV dysfunction | 318 | 44.1 | 28 | 22.6 | <0.001 |
| TAPSE > 20 mm | 233 | 32.7 | 53 | 43.4 | 0.021 |
| Dyspnea | |||||
| NYHA I | 2 | 0.3 | 1 | 0.8 | 0.23 |
| NYHA II | 49 | 6.8 | 11 | 8.9 | |
| NYHA III | 546 | 76.0 | 94 | 76.4 | |
| NYHA IV | 121 | 16.9 | 17 | 13.8 | |
| Laboratory results | |||||
| NT-proBNP (pg/mL) | 722 | 4246.5 (1980, 8330) | 124 | 2107.0 (1187, 4503) | <0.001 |
| Functional status | |||||
| 6-min walk distance | 213 | 226.3 ± 122.2 | 42 | 223.2 ± 104.1 | 0.78 |
| Baseline medication | |||||
| Beta blocker | 645 | 89.3 | 115 | 92.7 | 0.25 |
| ACEI or ARB or ARNI | 551 | 76.3 | 92 | 74.2 | 0.61 |
| MRA | 330 | 45.7 | 21 | 16.9 | <0.001 |
| Diuretic | 668 | 92.5 | 106 | 85.5 | 0.009 |
| Phenprocoumon | 183 | 25.3 | 30 | 24.2 | 0.78 |
| DOAK | 283 | 39.2 | 68 | 54.8 | 0.001 |
| Aspirin | 254 | 35.2 | 22 | 17.7 | <0.001 |
| Clopidogrel | 206 | 28.5 | 22 | 17.7 | 0.012 |
- Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; CRT, cardiac resynchronization therapy; DOAK, direct oral anticoagulant; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; RV, right ventricular; SD, standard deviation; sPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
The MitraScore was evaluated for all patients prior and postmitral TEER. However, the decrease of MitraScore points pre and post mitral TEER was comparable in the aFMR group compared to the vFMR group (vFMR: pre 9.5 ± 2.1 vs. post 2.7 ± 1.9 and MitraScore ≥4: pre 9.0 ± 2.1 vs. post 2.5 ± 1.8). All variables of the MitraScore in both groups pre and postmitral TEER are displayed in Table 2.
| vFMR | aFMR | |||||||
|---|---|---|---|---|---|---|---|---|
| n = 722 | n = 124 | |||||||
| Pre-TEER | Post-TEER | Pre-TEER | Post-TEER | |||||
| MR grading | n | % or Mean ± SD | n | % or Mean ± SD | n | % or Mean ± SD | n | % or Mean ± SD |
| MR trace | 0 | 0.0 | 227 | 31.4 | 0 | 0.0 | 47 | 37.9 |
| MR mild | 4 | 0.6 | 417 | 57.8 | 0 | 0.0 | 66 | 53.2 |
| MR moderate | 73 | 10.1 | 64 | 8.9 | 23 | 18.5 | 10 | 8.1 |
| MR severe | 645 | 89.3 | 14 | 1.9 | 101 | 81.5 | 1 | 0.8 |
| Eccentric Jet | 85 | 11.8 | 13 | 1.8 | 17 | 13.7 | 4 | 3.2 |
| Flow reversal PV | 107 | 14.8 | 5 | 0.7 | 12 | 9.7 | 1 | 0.8 |
| V-wave | ||||||||
| ≤20 mmHg | 114 | 15.8 | 316 | 43.8 | 19 | 15.3 | 63 | 50.8 |
| 22–34 mmHg | 309 | 42.8 | 364 | 50.4 | 67 | 54.0 | 53 | 42.7 |
| 35–50 mmHg | 225 | 31.2 | 38 | 5.3 | 30 | 24.2 | 7 | 5.6 |
| >50 mmHg | 74 | 10.2 | 4 | 0.6 | 8 | 6.5 | 1 | 0.8 |
| LV angiography | ||||||||
| MR trace | 0 | 0.0 | 197 | 27.3 | 1 | 1.3 | 43 | 34.7 |
| MR mild | 9 | 2.5 | 138 | 19.1 | 2 | 2.5 | 30 | 24.2 |
| MR moderate | 132 | 36.8 | 17 | 2.4 | 29 | 36.3 | 5 | 4.0 |
| MR severe | 212 | 59.1 | 1 | 0.1 | 45 | 56.3 | 0 | 0.0 |
| LVOT regurgitant bubbles to LA | ||||||||
| MR trace | 4 | 1.1 | 187 | 52.5 | 0 | 0.0 | 44 | 55.7 |
| MR mild | 6 | 1.7 | 139 | 39.0 | 5 | 6.3 | 30 | 38.0 |
| MR moderate | 143 | 39.9 | 28 | 7.9 | 28 | 35.0 | 5 | 6.3 |
| MR severe | 205 | 57.3 | 2 | 0.6 | 47 | 58.8 | 0 | 0.0 |
| 3D-EROA | ||||||||
| <0.1 cm2 | 0 | 0.0 | 103 | 27.6 | 0 | 0.0 | 10 | 20.8 |
| <0.2 cm2 | 6 | 1.6 | 145 | 38.9 | 1 | 2.0 | 28 | 58.3 |
| <0.3 cm2 | 26 | 6.9 | 65 | 17.4 | 6 | 12.0 | 7 | 14.6 |
| <0.4 cm2 | 39 | 10.4 | 23 | 6.2 | 8 | 16.0 | 1 | 2.1 |
| <0.5 cm2 | 58 | 15.4 | 20 | 5.4 | 11 | 22.0 | 2 | 4.2 |
| <0.6 cm2 | 45 | 12.0 | 7 | 1.9 | 4 | 8.0 | 0 | 0.0 |
| >0.6 cm2 | 202 | 53.7 | 10 | 2.7 | 20 | 40.0 | 0 | 0.0 |
| MitraScore | ||||||||
| Points | 722 | 9.5 ± 2.1 | 722 | 2.7 ± 1.9 | 124 | 9.0 ± 2.1 | 124 | 2.5 ± 1.8 |
- Abbreviations: EROA, effective regurgitation orifice area; LA, left atrium; LV, left ventricle; LVOT, left ventricular outflow tract; MR, mitral regurgitation; PV, pulmonary vein.
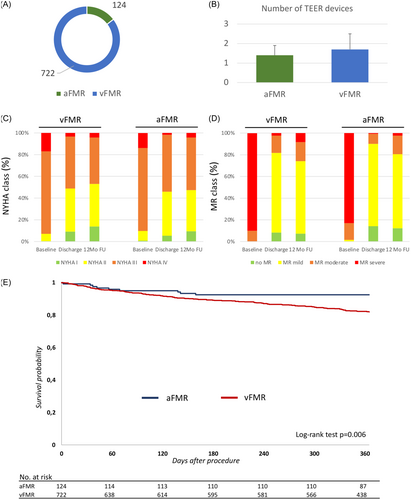
Significantly more TEER devices were used in vFMR compared to aFMR and also procedure time was significantly longer in patients with vFMR (Figure 2B, Table 3). Periinterventional complications were rare in both groups with a significant difference in postinterventional renal failure (vFMR 7.8% vs. aFMR 1.6%, p = 0.013). All procedural parameters are listed in Table 3.
| vFMR | aFMR | ||||
|---|---|---|---|---|---|
| n = 722 | n = 124 | p value | |||
| n | % or Mean ± SD | n | % or Mean ± SD | ||
| TEER devices | 722 | 1.7 ± 0.8 | 124 | 1.4 ± 0.5 | <0.001 |
| Procedure time | 722 | 68.0 (49, 90) | 124 | 60 (41.5, 86.5) | 0.019 |
| Complications | |||||
| Stroke | 3 | 0.4 | 1 | 0.8 | 0.55 |
| Myocardial infarction | 0 | 0.0 | 0 | 0.0 | |
| Pericardial tamponade | 2 | 0.3 | 1 | 0.8 | 0.36 |
| Renal failure | 56 | 7.8 | 2 | 1.6 | 0.013 |
| Dialysis | 24 | 3.3 | 2 | 1.6 | 0.30 |
| Major bleeding | 30 | 4.2 | 9 | 7.3 | 0.12 |
| Re-intervention/operation | 8 | 0.8 | 1 | 0.2 | 0.15 |
| In-hospital clinical course | |||||
| In-hospital mortality | 9 | 1.2 | 2 | 1.6 | 0.74 |
| MACCE (Death. Stroke, MI) | 12 | 1.7 | 3 | 2.5 | 0.55 |
| MACE (Death, MI) | 9 | 1.3 | 2 | 1.6 | 0.73 |
- Note: Major bleeding is defined as any bleeding requiring transfusion.
- Abbreviations: MACCE, major adverse cardiac and cerebrovascular events; MACE, major adverse cardiac events; MI, myocardial infarction; TEER, transcatheter edge-to-edge repair.
1-year clinical follow-up was completed in 695 patients (96.3%) of the vFMR group and 122 patients (98.4%) of the aFMR group. NYHA classification was comparable between aFMR and vFMR at baseline. In both groups, mitral TEER significantly improved NYHA classification at discharge and 12-months follow-up. No significant difference was found between both groups (Figure 2C). Also, MR classification assessed by transthoracic echocardiography showed comparable baseline values for aFMR and vFMR. Mitral TEER improved MR classification for both groups at discharge and 12-months follow-up. However, no significant difference between aFMR and vFMR was found (Figure 2D).
During the index procedure hospital stay, 9 patients of the vFMR group (1.2%) and 2 patients of the aFMR group (1.6%) died. Furthermore, the in-hospital MACE and MACCE rates were comparable between both groups (Table 3). 1-year mortality was significantly lower in patients with aFMR. Therefore, the mortality accounts for 7.5% in the aFMR group and 18.2% in the vFMR group (Figure 2E).
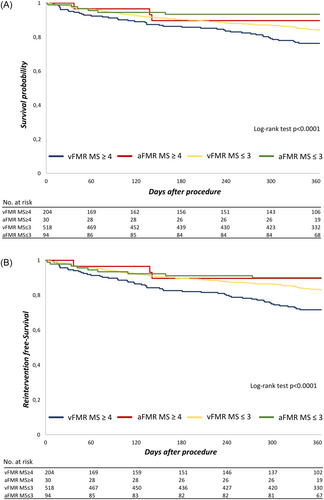
Patients with aFMR and vFMR were further allocated to MitraScore ≤3 and MitraScore ≥ 4. In the aFMR group, 94 patients showed a postprocedural MitraScore ≤3, while 30 patients had a MitraScore ≥4. Furthermore, in vFMR, 518 patients revealed a mild or no residual MR (MitraScore ≤3) and 204 patients showed a relevant residual MR (MitraScore ≥4) postmitral TEER. Baseline and procedural characteristics of the subgroups are shown in Table 4. In both subgroups 1-year mortality was significantly higher in the MitraScore ≥4 group (Figure 3A). However, 1-year mortality was significantly lower in the aFMR subgroups (aFMR: MitraScore ≤ 3 6.6% and MitraScore ≥ 4 10.3% vs. vFMR: MitraScore ≤ 3 16.1% and MitraScore ≥ 4 23.7%; Figure 3A). No significant difference was found for 1-year rehospitalization (aFMR: MitraScore ≤ 3 29.1% and MitraScore ≥ 4 46.2% vs. vFMR: MitraScore ≤ 3 38.8% and MitraScore ≥ 4 43.2%), while the combined end-point 1-year mortality and reintervention was significantly different between the groups (Figure 3B).
| vFMR | aFMR | |||
|---|---|---|---|---|
| MitraScore ≤ 3 | MitraScore ≥ 4 | MitraScore ≤ 3 | MitraScore ≥ 4 | |
| n = 518 | n = 204 | n = 94 | n = 30 | |
| % or Mean ± SD | % or Mean ± SD | % or Mean ± SD | % or Mean ± SD | |
| Age | 76 ± 9 | 75 ± 9 | 81 ± 5 | 80 ± 7 |
| Female | 35.1 (182/518) | 36.3 (74/204) | 70.2 (66/94) | 66.7 (20/30) |
| Echocardiography | ||||
| LVEF (%) | 35.0 ± 12.3 | 35.6 ± 14.0 | 58.0 ± 5.1 | 59.9 ± 5.4 |
| sPAP (mmHg) | 46.6 ± 14.0 | 49.2 ± 14.6 | 52.4 ± 32.6 | 46.6 ± 18.6 |
| MR Grading Baseline | ||||
| No MR | 0.0 (0/518) | 0.0 (0/204) | 0.0 (0/94) | 0.0 (0/30) |
| MR mild | 0.2 (1/518) | 0.0 (0/204) | 2.1 (2/94) | 0.0 (0/30) |
| MR moderate | 11.4 (59/518) | 5.9 (12/204) | 19.1 (18/94) | 3.3 (1/30) |
| MR severe | 88.4 (458/518) | 94.1 (152/234) | 78.7 (74/94) | 96.7 (29/30) |
| Dyspnea baseline | ||||
| NYHA I | 0.4 (2/515) | 0.0 (0/203) | 1.1 (1/94) | 0.0 (0/29) |
| NYHA II | 7.0 (36/515) | 6.4 (13/203) | 10.6 (10/94) | 3.4 (1/29) |
| NYHA III | 74.8 (385/515) | 79.3 (161/203) | 72.3 (68/94) | 89.7 (26/29) |
| NYHA IV | 17.9 (92/515) | 14.3 (29/203) | 16.0 (15/94) | 6.9 (2/29) |
| Laboratory results | ||||
| NT-proBNP (pg/mL) | 4121 (1914, 7503) | 4647.5 (2230, 10573) | 2171 (1158, 4503) | 1957 (1201, 5030) |
| MitraScore | ||||
| Points pre-TEER | 9.2 ± 2.1 | 10.3 ± 1.6 | 8.6 ± 2.2 | 10.3 ± 1.5 |
| Points post-TEER | 1.8 ± 1.0 | 5.2 ± 1.5 | 1.8 ± 1.1 | 4.9 ± 1.2 |
| Procedure | ||||
| TEER devices | 1.7 ± 0.7 | 1.8 ± 0.8 | 1.4 ± 0.6 | 1.3 ± 0.5 |
| 1-year follow-up | ||||
| Mortality | 16.1 | 23.7 | 6.6 | 10.3 |
| Death/re-intervention | 17.1 | 28.3 | 9.9 | 10.3 |
| Rehospitalization | 38.8 | 43.2 | 29.1 | 46.2 |
| MR Grading 1-year FU | ||||
| No MR | 6.5 (9/139) | 9.4 (5/53) | 10.3 (3/29) | 16.7 (2/12) |
| MR mild | 69.8 (97/139) | 58.5 (31/53) | 72.4 (21/29) | 58.3 (7/12) |
| MR moderate | 17.3 (24/139) | 18.9 (10/53) | 13.8 (4/29) | 25.0 (3/12) |
| MR severe | 6.5 (9/139) | 13.2 (7/53) | 3.4 (1/29) | 0.0 (0/12) |
| Dyspnea 1-year FU | ||||
| NYHA I | 14.0 (49/351) | 13.2 (16/121) | 11.3 (8/71) | 4.2 (1/24) |
| NYHA II | 40.5 (142/351) | 35.5 (43/121) | 40.8 (29/71) | 29.2 (7/24) |
| NYHA III | 41.9 (147/351) | 45.5 (55/121) | 45.1 (32/71) | 58.3 (14/24) |
| NYHA IV | 3.7 (13/351) | 5.8 (7/121) | 2.8 (2/71) | 8.3 (2/24) |
| Laboratory results 1-year FU | ||||
| NT-proBNP (pg/mL) | 2743.5 (1358, 6190.5) | 2634 (1279, 5503.5) | 905 (635, 1874) | 1716 (1364, 2280) |
- Abbreviations: FU, follow-up; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; NYHA, New York Heart Association; SD, standard deviation; sPAP, systolic pulmonary artery pressure; TEER, transcatheter edge-to-edge repair.
Residual MR (MITRA-Score ≥ 4) was an independent predictor of survival in a multivariate analysis in all FMR patients (n = 846) with a HR (95%): 1.57 (1.09–2.25). Other independent predictors of 1-year mortality were impaired renal function (eGFR < 30 mL/min/1.73 m2) and LVEF. Furthermore, we found a MITRA-Score ≥ 4 to be an independent predictor of 1-year mortality in vFMR (n = 722) with a HR (95%): 1.58 (1.09–2.30). Impaired renal function (eGFR < 30 mL/min/1.73 m2) was also associated with 1-year survival with a HR (95%): 1.94 (1.31–2.88) in vFMR. In aFMR, where the number of patients (n = 122) and the event rate (n = 9) were low, residual MR was statistically no independent predictor of mortality with a HR (95%): 0.89 (0.19–4.22). Of note, no parameter of the multivariate analysis in aFMR was found to be an independent predictor of mortality indicating a low statistical power due to the low event rate in this cohort.
4 DISCUSSION
This analysis of the large-scale multicenter MITRA-PRO registry describes for the first time the impact of residual MR on 1-year survival and rehospitalization in aFMR versus vFMR. Recently feasibility and efficacy of mitral TEER in the context of aFMR were demonstrated by an analysis of the EuroSMR registry.4 These effects and clinical improvement were found to be comparable to patients with vFMR.4 The prevalence of aFMR was 7.8% in this highly selected TEER cohort. In our analysis we found a comparable prevalence of 8.3%, which is also in line with other studies like the EXPAND trial (12.8%), MITRA-TUNE registry (7.5%) or GIOTTO (6.0%).5, 7, 11 Patients with aFMR share typical characteristics compared to vFMR: (1) more female patients, (2) higher mean age, (3) more arterial hypertension and (4) less coronary artery disease. These findings are related to the underlying HFpEF in patients with aFMR. TEER in aFMR showed a good safety profile with a low in-hospital MACCE rate of 2.5%, which is in line with previous publications such as GIOTTO registry (complication rate 2%), MITRA-TUNE (in hospital death 2%) or a Spanish registry (in-hospital death 2.1%).7, 11, 12
In the MITRA-TUNE registry NYHA stage III/IV was found in 35/65% at baseline and 0/10% at follow-up and in the EXPAND trial in 11.3/73.6% at baseline and also 0/21.1% at follow-up of the aFMR patients.5, 7 In the MITRA-PRO registry we found NYHA stage III/IV in 13.8/76.4% at baseline and in 4.2/48.4% at 12-months follow-up, which is significantly higher than reported for aFMR. Of note, in the MITRA-PRO registry follow-up clinical assessment was done investigator-independent from a central side to exclude investigator bias. This might influence the NYHA stage, since investigator-dependent assessment tends to report better results. Therefore, comparison of functional outcome using NYHA classification is limited by the method of assessment and structured heart failure questionnaires are more reliable. However, as previously published we confirmed that functional outcome is improved by mitral TEER in both aFMR and vFMR. Furthermore, we found no significant difference in NYHA classification stage III/IV between vFMR and aFMR at 12-months follow-up. Also, dyspnea is multifactorial and the underlying cause of aFMR and vFMR might influence functional outcome.
One-year mortality was significantly lower in patients with aFMR compared to vFMR. 1-year survival accounts for 92.5% in the aFMR group and 81.8% in the vFMR group. Other studies reported no significant difference in mortality between aFMR und vFMR following mitral TEER.4 However, both a prospective international multi-ethnic cohort study and a metanalysis of current studies showed, that HFpEF has a better long-term survival than HFrEF, which strengthens our findings for aFMR and vFMR patients.13, 14
Definition of aFMR is still a matter of debate and inclusion criteria vary among the studies.2, 15 Previous trials included only patients with AF in the aFMR group,5, 7, 12 while other also included HFpEF patients without AF.4, 11 The absence of diagnosed AF per se did not exclude aFMR, since LA dilatation could also be found in patients with sinus rhythm or in the presence of asymptomatic, not yet diagnosed paroxysmal AF. In the MITRA-PRO registry the aFMR group showed a history of AF in 83%, while AF was only assessed by the medical history and baseline or follow-up ECG. Therefore, one might speculate an underestimated rate of AF in the aFMR cohort. However, no adequate AF screening was performed in the MITRA-PRO registry. Therefore, AF was not used as an inclusion criterion for the aFMR group, which is in line with current reviews.2, 15
- 1.
aFMR Patients with mild residual MR (MitraScore ≤ 3) had a lower mortality rate (6.6% vs. 10.3%) and rehospitalization rate 1 year after TEER (29.1% vs 46.2%). However, in contrast to vFMR a MitraScore ≥ 4 was no independent predictor of survival in aFMR.
- 2.
compared to vFMR, similar procedural results in regard of residual MR following TEER were found in aFMR patients (MitraScore post TEER 2.5 ± 1.8 vs. 2.7 ± 1.9), while the amount of implanted TEER devices was increased in vFMR.
- 3.
1-year survival was better in aFMR compared to vFMR regardless of relevant residual MR (MitraScore ≥ 4), while 1-year rehospitalization was comparable for both MR entities.
Residual MR assessed by the MitraScore was an independent predictor of 1-year mortality in all FMR (n = 846; aFMR + vFMR) and vFMR (n = 722) patients. In aFMR, a MitraScore of ≥4 was associated with a higher mortality rate in univariate analysis. However, it was not an independent predictor of survival in multivariate analysis, likely due to the low event rate in this cohort. These findings highlight the critical role of LV function in overall survival for heart failure patients. Consequently, in vFMR patients, significant residual MR substantially impacts the 1-year mortality rate, whereas aFMR patients exhibit better tolerance to residual MR following mitral TEER. One might speculate that long-term follow-up (3 and 5 years) of the MITRA-PRO registry, which is not yet available, may reveal a MitraScore of ≥4 as an independent predictor of mortality following mitral TEER. However, at the 1-year follow-up, the MITRA-PRO registry only indicated a trend toward a higher mortality rate for aFMR patients with relevant residual MR. However, minimizing MR through mitral TEER to the lowest possible level is crucial for survival, especially in vFMR patients. In contrast, aFMR patients show better tolerance to significant residual MR 1 year after the initial procedure.
5 STUDY LIMITATIONS
Assessment of residual MR was done by MITRA-PRO investigators and investigator bias cannot be excluded. Furthermore, in this real-world registry guideline directed medical treatment (GDMT) was not controlled at 1-year follow-up. Therefore, changes in GDMT might have influenced the clinical outcome. Of note, the MITRA-PRO registry was designed before publication of COAPT and MITRA-FR study results and right ventricular function and LV dimensions were not considered for patient selection. MITRA-PRO included patients from 2016 to 2020, a period during which the novel MitraClip G4 was not yet available. This absence could potentially influence the incidence of residual MR. Furthermore, long-term follow-up (3 and 5 years) is yet not available and will provide further evidence of the impact of residual MR following TEER on long-term survival especially in patients with aFMR, where the event rate was low at 1-year follow-up. Logistic EuroScore, which is known to be an overpredictive risk model, was used in MITRA-PRO for risk assessment. EuroScore II or STS score would have been more accurate, but were not part of the study protocol designed in 2014. Mitral annular dimensions were not assessed in the MITRA-PRO registry and were not part of the inclusion criteria for aFMR, while LA dimensions were used to allocate patients to the aFMR group. AF might have influenced mortality of patients with aFMR and vFMR and was not assessed during the clinical follow-up. However, monitoring AF in clinical trials remains challenging, since ECG or even holter monitoring during follow-up might underestimate the burden of AF.
6 CONCLUSIONS
This MITRA-PRO registry analysis showed better 1-year survival in aFMR compared to vFMR regardless of relevant residual MR, while 1-year rehospitalization rates were comparable for both MR entities. Furthermore, aFMR patients had similar procedural outcomes regarding residual MR following TEER compared to vFMR patients, while the amount of implanted TEER devices was increased in vFMR. Residual MR is an independent predictor of 1-year mortality in vFMR patients, whereas in aFMR patients, a MitraScore of ≥4 is associated with higher mortality but is not an independent predictor in multivariate analysis. Therefore, minimizing MR through mitral TEER is crucial for survival in vFMR patients, while aFMR patients tolerate significant residual MR better 1 year after the procedure.
ACKNOWLEDGMENTS
Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.