First-in-human: Leaflet laceration with balloon mediated annihilation to prevent coronary obstruction with radiofrequency needle (LLAMACORN) for valve-in-valve transcatheter aortic valve replacement
Abstract
Coronary obstruction (CO) is a potential pitfall for transcatheter aortic valve replacement (TAVR), especially in valve in valve procedures into degenerated surgical or transcatheter prostheses. Bioprosthetic leaflet modification techniques that incorporate electrosurgery are evolving as the preferred strategy to mitigate the risk of CO in high CO risk settings. The UNICORN method is proposed as a more predictable leaflet modification strategy than the earlier described BASILICA approach, but its proponents have hitherto mandated the use of a balloon-expandable valve (BEV) prosthesis. Many patients have small prostheses and therein face a significant risk of patient prosthesis mismatch with BEV in this setting. This risk may be curtailed if a self-expanding valve (SEV) prosthesis could be used. Herein described is a modified approach to allow for the utilization of SEV systems in this setting.
1 INTRODUCTION
Coronary obstruction (CO) may complicate transcatheter aortic valve replacement (TAVR) as native or bioprosthetic aortic valve leaflets are displaced toward the coronary artery ostia during transcatheter valve deployment.1 This is a rare but potentially life-threatening complication with an incidence of <1% and 30-day mortality of 41%.2 Risk factors for CO include female gender, low coronary height, small sinus of Valsalva, those receiving balloon-expandable valves (BEV), and those with previous surgical aortic bioprostheses (SAVR).2, 3
Prophylactic strategies have been described to mitigate CO risk. These include coronary wire protection,4 prophylactic chimney-snorkel stenting5, 6 and novel transcatheter electrosurgical leaflet modification techniques, specifically BASILICA (Bioprosthetic or Native Aortic Scallop Intentional Laceration to Prevent Iatrogenic Coronary Artery Obstruction)1 and UNICORN (Undermining Iatrogenic CO with Radiofrequency Needle).7 The premise of the electrosurgical approaches is to modify and splay aortic leaflets to preserve coronary artery flow post TAVR.8 Both BEV and self-expanding (SEV) transcatheter heart valves (THV) have been used for BASILICA facilitated TAVR.9 The developers of the UNICORN technique have mandated the use of BEV as SEV platforms were considered to have insufficient radial strength to splay the leaflet after initial ballooning. However, in many patients where UNICORN may be efficacious including for TAVR-in-SAVR and TAVR-in-TAVR, there is a significant risk of patient-prosthesis mismatch (PPM) with BEV owing to small original prosthesis size.10 Further, TAVR-in-TAVR with BEV may have suboptimal performance if there is supra-annular leaflet overhang from the original SEV.11 Via iterative modifications to the UNICORN technique for BEV, we describe our consecutive case experience of using sequential leaflet modification measures to facilitate safe use of SEV in these settings. To allow for this distinction this modified technique has been labeled LLAMACORN (Leaflet Laceration with bAlloon Mediated Annihilation to prevent CO with Radiofrequency Needle).
2 CASE PRESENTATIONS
2.1 Patients
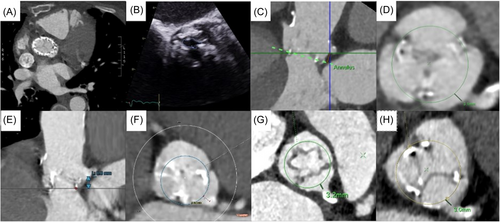
We present a case series of five patients. Their clinical presentations and imaging findings are summarized in Table 1 and Figure 1. One patient was a TAVR-in-TAVR and the remaining four cases were TAVR-in-SAVR into Trifecta (Abbott) or Mitroflow (Sorin) prostheses (externally mounted bioprosthetic leaflets). Owing to their various comorbidities, all five patients were deemed high to prohibitive risk for open surgery at multidisciplinary heart team assessment.
| Case # | Age/sex | Case presentation | Echocardiogram | Cardiac Computed Tomography |
|---|---|---|---|---|
| 1 | 69/ F |
NYHA IV; early failure of 29 mm Evolut Pro+ (Medtronic) implanted into a 23 mm native aortic annulus at another center. | 4+ transvalvular AR, LVEF 65%, DVI 0.58. | HALT likely due to a constrained valve with leaflet pinwheeling and altered flow dynamics. Persistence of findings despite three months of anticoagulation. High risk of LMCA CO from leaflet obstruction and sinus sequestration. |
| 2 | 83/ F |
NYHA III dyspnea and CCS III angina; degenerative 21 mm Trifecta (Abbott) aortic prosthesis. | Severe AS, LVEF 65%, mAVG 48 mmHg, DVI 0.20, 2D planimetry of bioprosthetic aortic valve orifice 0.41cm2. | LMCA height 6.4 mm, VTC 3.1 mm. |
| 3 | 84/ F |
NYHA III; degenerative 19 mm Trifecta (Abbott) aortic prosthesis. | Severe AS, LVEF 58%, mAVG 36 mmHg, DVI 0.2, EOA 0.5cm2; 3+ transvalvular AR. | LMCA height 8.8 mm, VTC 3.8 mm. |
| 4 | 87/ M |
NYHA IV; degenerative 25 mm Mitroflow (Sorin) aortic prosthesis. | 4+ transvalvular AR, LVEF 45%, DVI 0.52. | LMCA height 10.0 mm, VTC 3.2 mm. |
| 5 | 84/ M |
NYHA III dyspnoea; degenerative 21 mm Trifecta (Abbott) aortic prosthesis. | 4+ transvalvular AR, LVEF 60%, DVI 0.40. | LMCA height 8.5 mm, VTC 3.0 mm. |
- Abbreviations: AR, aortic regurgitation; AS, aortic stenosis; CCS, Canadian Cardiovascular Society; DVI, dimensionless velocity index; HALT, hypoattenuation with leaflet thickening; LMCA, left main coronary artery; NYHA, New York Heart Association.
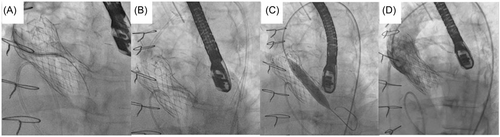
2.2 Procedural set-up
The procedures were performed under general anesthesia in the cardiac catheterization laboratory with fluoroscopic and transoesophageal echocardiogram (TEE) guidance. Intravenous unfractionated heparin was administered to an activated clotting time of >300 s. Ultrasound guided arterial access with Prostyle (Abbot) preclose technique to the right femoral artery with sequential upsizing to a 12−14 F sheath over an Amplatz Super Stiff wire (Boston Scientific). Ultrasound guided venous access to the right femoral vein with a 6 F sheath and a 5 F J balloon tipped temporary pacing electrode positioned in the right ventricle. Contralateral access for aortogram was either via left femoral or right radial artery with a 6 F sheath.
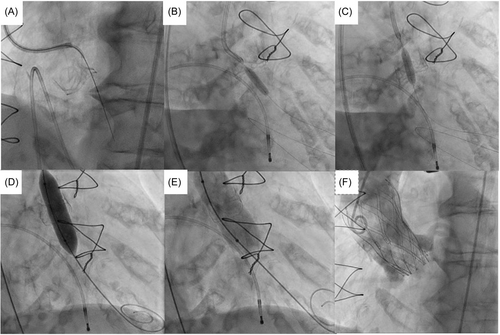
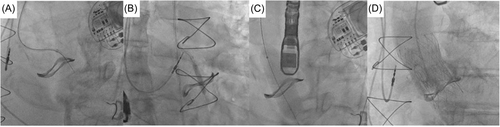
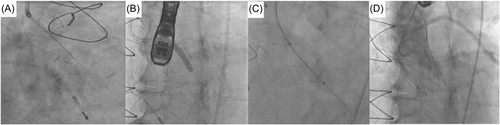
2.3 Leaflet penetration and sequential ballooning
In all five cases, penetration of the base of the left coronary cusp was achieved with an Astato XS 40 300 cm 0.014” wire (Asahi) with cautery set to 100 W pure-cut mode. This wire was delivered via a telescopic system outlined in Table 2. Following fluoroscopic and TEE confirmation of leaflet traversal, a 330 cm RG3 wire (Asahi) was exchanged via the microcatheter with the distal end allowed to loop. Over this newly created aorto-ventricular rail through the penetrated left bioprosthetic aortic valve leaflet, sequential ballooning was then performed to achieve leaflet perforation (see Table 2). Following initial ballooning with coronary balloons over the RG3, a 110 cm 5 F pigtail catheter was exchanged over the RG3 into the LV and then this allowed positioning of a pre-shaped 275 cm 0.035” extra-small TAVR guidewire (Safari2, Boston Scientific) through the hole in the leaflet with sequential ballooning with vascular and then aortic valvuloplasty sized balloons for inside-out leaflet annihilation.
| Case # | Delivery telescopic system for leaflet penetration | Sequential ballooning for leaflet perforation | THV | Postdilatation |
|---|---|---|---|---|
| 1 | 8 F Extra-backup (EBU) 3.5 guide catheter (Medtronic), 5 F cut pigtail diagnostic catheter, 130 cm 1.8 F Finecross microcatheter (Terumo). |
Mini Trek 2.0 x 15 mm coronary balloon (Abbott), Mustang over the wire 10 x 40 mm peripheral vascular balloon (Boston Scientific), Cristal 18 x 40 mm balloon (Balt) | Navitor 27 mm (Abbott) | Cristal 25 x 50 mm balloon (Balt) |
| 2 | 7 F EBU 3.0 guide catheter (Medtronic), 5 F cut pigtail diagnostic catheter, 130 cm 1.8 F Finecross microcatheter (Terumo). |
Mini Trek 1.5 x 20 mm coronary balloon (Abbott), Trek 4.0 x 20 mm coronary balloon (Abbott), Valver 18 x 40 mm balloon (Balton). | Navitor 23 mm (Abbott) | True 22 x 45 mm balloon (BD |
| 3 | 7 F Extra-backup (XB) 3.0 guide catheter (Cordis), 5 F cut pigtail diagnostic catheter, 130 cm 1.8 F Finecross microcatheter (Terumo). |
Trek 4.5 x 15 mm coronary balloon (Abbott), NC Emerge 6.0 x 12 mm coronary balloon (Boston Scientific), Armada 14 x 40 mm peripheral vascular balloon (Abbott), Valver 18 x 40 mm balloon (Balton). | Navitor 23 mm (Abbott) | True 22 x 45 mm balloon (BD) |
| 4 | 7 F Amplatz left (AL) 1 guide catheter (Medtronic), 5 F cut pigtail diagnostic catheter, 130 cm 1.8 F Finecross microcatheter (Terumo). |
Mini trek 2.5 x 15 mm coronary balloon (Abbott), Trek 4.0 x 20 mm coronary balloon (Abbott), Valver 18 x 40 mm balloon (Balton). | Navitor 25 mm (Abbott) | Atlas Gold 24 x 40 mm balloon (BD) |
| 5 | 7 F Extra-Backup (EBU) 3.0 guide catheter (Medtronic), 5 F cut pigtail diagnostic catheter, 130 cm 1.8 F Finecross microcatheter (Terumo). |
Mini trek 2.5 x 20 mm coronary balloon (Abbott), Trek 4.0 x 20 mm coronary balloon (Abbott), Cristal 18 x 40 mm balloon (Balt). | Navitor 23 mm (Abbott) | Atlas Gold 22 x 40 mm balloon (BD) |
- Abbreviations: LLAMACORN, Leaflet Laceration with bAlloon Mediated Annihilation to prevent CO with Radiofrequency Needle; THV, transcatheter heart valve.
2.4 Valve deployment and procedural outcome
The THV was deployed under controlled ventricular pacing at 120–140 bpm and postdilatation performed in all cases under rapid ventricular pacing to 180 bpm. Angiography confirmed patency of coronary arteries, with TEE and hemodynamic assessment confirming no to trivial aortic regurgitation (see Figures 2-6).
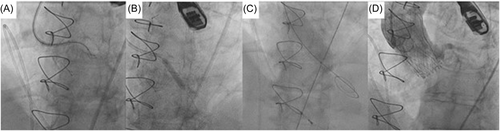
2.5 30-day follow-up
All five cases had a successful uncomplicated TAVR with no CO and no conduction abnormalities necessitating permanent pacemaker implantation, discharged to home and were alive at 30-day follow-up. No patient suffered stroke or vascular complications. The echocardiographic findings are shown in Table 3 with satisfactory hemodynamic parameters.
| Case number | mAVG (mmHg) | DVI | EOA (cm2) | Aortic regurgitation |
|---|---|---|---|---|
| 1 | 11 | 0.56 | 1.8 | Trivial paravalvular |
| 2 | 11 | 0.45 | 1.5 | None |
| 3 | 10 | 0.30 | 1.0 | Mild paravalvular |
| 4 | 5 | 0.68 | 1.8 | Trivial paravalvular |
| 5 | 21 | 0.44 | 1.5 | None |
- Abbreviations: DVI, dimensionless velocity index; EOA, effective orifice area; mAVG, mean aortic valve gradient; TTE, transthoracic echocardiogram.
3 DISCUSSION
This case series illustrates the potential for LLAMACORN to allow for TAVR-in-SAVR or TAVR-in-TAVR with SEV prostheses at high risk for CO. In case 1, there were two issues to contend with for a TAVR-in-TAVR procedure. First, it has been suggested that BASILICA for a degenerative SEV may not provide adequate leaflet splaying to prevent CO.12 Second, UNICORN would normally have mandated the use of BEV, and inside a large supra-annular SEV there was concern for potential leaflet overhang from the SEV. In cases 2 to 5 the failed aortic bioprostheses had externally mounted leaflets which are associated with higher risk of CO. The use of SEV for these cases was to try and minimize the likelihood of PPM, which would be more likely to arise with a BEV into the small SAVR prostheses.13, 14 LLAMACORN allowed for the successful management of these issues in all cases with satisfactory clinical and hemodynamic findings at follow-up.
4 CONCLUSION
LLAMACORN represents a potential alternative as a modification of UNICORN to facilitate the use of SEV for valve in valve procedures. Further investigation of LLAMACORN appears warranted.
ACKNOWLEDGMENTS
Open access publishing facilitated by The University of Queensland, as part of the Wiley - The University of Queensland agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST STATEMENT
Anthony C. Camuglia and Christopher M. W. Cole are clinical proctors for Edwards Lifesciences. A. C. C. M., Christopher M. W. Cole, Samual M. Hayman and Stephen V. Cox are clinical proctors for Abbott. All authors work for institutions who have received research support from Abbott, Medtronic and Boston Scientific. The remaining authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.