Mechanical circulatory support versus vasopressors alone in patients with acute myocardial infarction and cardiogenic shock undergoing percutaneous coronary intervention
Abstract
Background
Previous studies have compared Impella use to intra-aortic balloon pump (IABP) use in patients with acute myocardial infarction and cardiogenic shock (AMI-CS) undergoing percutaneous coronary intervention (PCI). Our objective was to compare clinical outcomes in patients with AMI-CS undergoing PCI who received Impella (percutaneous left ventricular assist device) without vasopressors, IABP without vasopressors, and vasopressors without mechanical circulatory support (MCS).
Methods
We queried the National Inpatient Sample (NIS) using ICD-10 codes (2015–2018) to identify patients with AMI-CS undergoing PCI. We created three propensity-matched cohorts to examine clinical outcomes in patients receiving Impella versus IABP, Impella versus vasopressors without MCS, and IABP versus vasopressors without MCS.
Results
Among 17,762 patients, Impella use was associated with significantly higher in-hospital major bleeding (31.4% vs. 13.6%; p < 0.001) and hospital charges (p < 0.001) compared to IABP use, with no benefit in mortality (34.1% vs. 26.9%; p = 0.06). Impella use was associated with significantly higher mortality (42.3% vs. 35.7%; p = 0.02), major bleeding (33.9% vs. 22.7%; p = 0.001), and hospital charges (p < 0.001), when compared to the use of vasopressors without MCS. There were no significant differences in clinical outcomes between IABP use and the use of vasopressor without MCS.
Conclusions
In this analysis of retrospective data of patients with AMI-CS undergoing PCI, Impella use was associated with higher mortality, major bleeding, and in-hospital charges when compared to vasopressor therapy without MCS. When compared to IABP use, Impella was associated with no mortality benefit, along with higher major bleeding events and in-hospital charges. A vasopressor-only strategy suggested no difference in clinical outcomes when compared to IABP. This study uses the NIS for the first time to highlight outcomes in AMI-CS patients undergoing PCI when treated with vasopressor support without MCS, compared to Impella and IABP use.
Abbreviations
-
- AMI
-
- acute myocardial infarction
-
- CAD
-
- coronary artery disease
-
- CKD
-
- chronic kidney disease
-
- COPD
-
- chronic obstructive pulmonary disease
-
- CS
-
- cardiogenic shock
-
- ESRD
-
- end stage renal disease
-
- IABP
-
- intra-aortic balloon pump
-
- IQR
-
- interquartile range
-
- NSTEMI
-
- non-ST-elevation myocardial infarction
-
- PCI
-
- percutaneous coronary intervention
-
- SMD
-
- standardized mean difference
-
- STEMI
-
- ST-elevation myocardial infarction
-
- TIA
-
- transient ischemic attack
1 INTRODUCTION
Acute myocardial infarction (AMI) complicated by concomitant cardiogenic shock (CS) is associated with a mortality rate of approximately 50%.1, 2 In this population, short-term mechanical circulatory support (MCS) can be used to improve cardiac output, end-organ perfusion, and hypoxemia.3 The intra-aortic balloon pump (IABP) and the Impella percutaneous left ventricular assist device (Abiomed) are frequently used for patients requiring MCS.4 Despite the increasing use of MCS devices in patients with AMI and CS (AMI-CS), a standardized approach for the type of MCS device and timing of placement is absent.3 MCS choice often varies with regional practice patterns and operator preference. Although there is a reinvigorated movement to pursue MCS in AMI-CS patients based on objective criteria with an emphasis on an early time-to-support philosophy,5-10 current guidelines lack robust data to guide selection for the type of MCS device to use, and no randomized controlled trials (RCTs) clearly support MCS use in AMI-CS.3 IABP use in patients with AMI-CS was questioned after the results of the IABP-SHOCK II trial, due to lack of benefit in short and long-term mortality. As a result, IABP use for AMI-CS began to decrease.4 In Europe, IABP use has steeply declined, as the 2016 and 2021 European Society of Cardiology guidelines for the diagnosis and treatment of acute and chronic heart failure have given the routine use of IABP in AMI-CS a Class IIIB recommendation.3, 11 The same guidelines give a Class IIb recommendation for IABP as a bridge to other therapies and Class IIa recommendation for short-term MCS in CS as a bridge to other therapies, without specifying the choice of MCS device.3 American College of Cardiology guidelines for the management of ST-elevation myocardial infarction (STEMI) give a Class IIa recommendation for IABP and IIb recommendation for alternative left ventricular assist devices in patients with CS after STEMI who do not quickly stabilize with pharmacological therapy.12
Nonetheless, the IABP is still used to maintain coronary perfusion pressure and support hemodynamics in high-risk coronary anatomy as a temporizing measure and its use in AMI-CS remains ubiquitous worldwide. Impella use has substantially increased in the last decade due to the improved hemodynamic profile it provides compared to the IABP, although RCTs of Impella versus IABP in high-risk percutaneous coronary intervention (PCI) or management of AMI-CS have not found Impella to be superior with respect to mortality.13, 14 In Europe, the DanGer Shock Trial, an RCT evaluating Impella compared to conventional guideline-driven treatment including vasopressors, is ongoing, but results are not yet available.1
Prior large retrospective studies of AMI-CS patients using NIS or other databases have focused on comparing clinical outcomes between Impella and IABP and did not include direct comparisons of MCS to vasopressor support or adjust for lactic acidosis, a well-known prognostic biomarker in CS.15-17 In current clinical practice, there is often an assumption that the standard of care for AMI-CS patients undergoing PCI is MCS based on its widespread use, although there is no randomized data to support this notion.4 Therefore, our objective was to compare clinical outcomes in patients with AMI-CS undergoing PCI during the same hospitalization, who received Impella without vasopressors or other MCS, IABP without vasopressors or other MCS, or vasopressor therapy without MCS, using the most recent data from a large public database. We aimed to adjust for severity of hypoperfusion by the presence of lactic acidosis and acuity of illness using propensity-matched analysis of four relevant clinical variables.
2 METHODS
2.1 Study data source
The data source for this study was the United States (US) National Inpatient Sample (NIS), from October 1, 2015, to December 31, 2018. The NIS database is part of the Healthcare Cost and Utilization Project (HCUP) databases and is sponsored by the Agency for Healthcare Research and Quality (AHRQ). The NIS is the largest publicly available all-payer administrative claims-based database and contains patient discharges from 1000 hospitals in 45 states. It has clinical and resource utilization information on more than 7,000,000 discharges annually. When weighted, it represents more than 35,000,000 national hospitalizations annually. These data are randomly selected to approximately represent a 20% sample of US inpatient hospitalizations across different hospitals and geographic regions. This study was approved by The University of Texas Health at San Antonio Institutional Review Board and received proper ethical oversight. Informed consent was not required for this study as the data were deidentified. Details of data elements, sampling design, coding, and purchasing can be found on www.hcup.org.
2.2 Study population
Patients in a domain comprised of those with CS and AMI undergoing PCI during the same hospital admission were included in the study. Patients were identified using the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). ICD-10 codes used to identify patients are detailed in the Supplementary Material (Supporting Information: Tables S1–S4). Interventions to be compared were defined as Impella use without other MCS or vasopressors, IABP use without other MCS or vasopressors, and vasopressor use without MCS. Vasopressor includes the possible use of inotropes in the NIS database, as there is no separate code for inotropes. Patients receiving both MCS and vasopressors were excluded. We chose to exclude patients receiving extracorporeal membrane oxygenation with no other MCS, as this subgroup had too few patients to make robust statistical comparisons. Our main aim was to compare clinical outcomes in patients with AMI-CS receiving Impella without vasopressors, IABP without vasopressors, and vasopressors without MCS. In this manner, we intended to isolate the effect of each intervention.
2.3 Study outcomes
The primary outcome of the study was in-hospital mortality. Key secondary outcomes included major bleeding, total length of hospital stay (LOS) measured in days, and total hospital charges measured in US dollars. Additional outcomes are summarized in Tables 4–6.
2.4 Statistical methods
Impella versus IABP, Impella versus vasopressor therapy without MCS, and IABP versus vasopressor therapy without MCS were compared without adjustment for inpatient diagnoses, demographics, and medical history variables and after propensity score pairwise matching with adjustment for 24 variables. A nearest-neighbor 1:1 greedy matched, parallel, balanced propensity-matching model was applied using a caliper width of 0.005. The clinical variables in the propensity model included in-patient diagnosis of lactic acidosis, acute kidney injury and hypotension. Non-ST elevation myocardial infarction (NSTEMI) was used to match patients to balance the type of acute coronary syndrome (NSTEMI and STEMI) across cohorts. Demographic variables used in the propensity model included age, race and payer status. Comorbidities in patients’ previous medical history used in the propensity model included diabetes, hypertension, hyperlipidemia, CAD, peripheral artery disease, obesity, smoking, cerebrovascular accident or transient ischemic attack, PCI, valvular heart disease, atrial fibrillation, smoking, chronic obstructive pulmonary disease, anemia, chronic kidney disease stages 3–5 and end-stage renal disease. Matching was conducted on the logit of the propensity score with exact matching on the domain indicator. Outcome analyses were conducted with adjustment for the same covariates included in the propensity model with a conditional logistic regression model for matched pairs adjusted for hospital clustering, with a domain of patients with CS and acute MI who underwent PCI while in hospital, stratified by NIS strata and the match set identifier, and weighted with HCUP survey sample weights provided by AHRQ. Data were summarized for demographic characteristics with percentages and absolute standardized mean differences (SMD) and for clinical outcomes with percentages, odds ratios, 95% confidence intervals for the odds ratios, and p value. Published literature provides no universal agreement on a definition for a small SMD; however, an often cited and accepted definition of small SMD is 0.25 or less.18 For outcome analysis, hospital size was defined as large if the yearly number of hospital discharges was greater than or equal to the yearly median number of discharges per year in the population of hospitals and small otherwise. All statistical testing was two-sided with a significance level of 5%. Corrections for multiple comparisons were not applied. SAS version 9.4 for Windows (SAS Institute) was used throughout. Statistically significant outcomes are described, and nonsignificant results are briefly summarized.
3 RESULTS
A total of 115,882,699 hospitalizations between October 2015 to December 2018 were analyzed (Figure 1). After limiting the domain to patients with CS and acute MI who underwent PCI while inpatient (n = 84,699), 17,762 patients met the criteria, of which 14,069 (79.2%) were in the Impella group, 1349 patients in the IABP group (7.5%), and 2344 (13.1%) were in the vasopressor group without MCS. We created three separate propensity-matched pair cohorts to evaluate the effect of Impella versus vasopressors without MCS (2269 versus 2269), Impella versus IABP (1320 versus 1320), and IABP versus vasopressors with no MCS (904 versus 904).
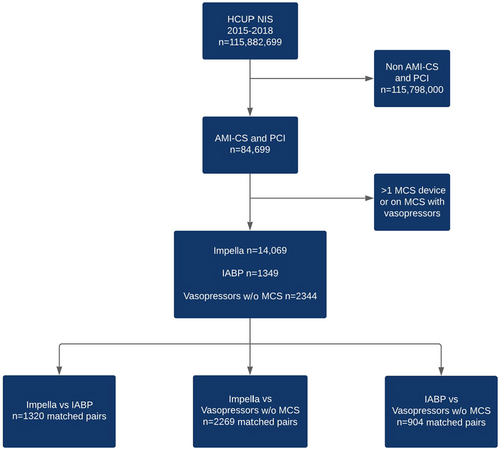
The baseline characteristics of the unmatched study cohorts are displayed in Tables 1–3. The median age in the Impella cohort was 66 years (interquartile range [IQR] 57–73), in the IABP cohort 64 years (IQR 57–76), and in the vasopressors without MCS cohort 66 years (IQR 58–75). A large portion of patients were male: 71.7% (Impella), 65.6% (IABP), and 67% (vasopressors without MCS). A majority of patients had history of CAD, were of White race, and had Medicare as insurance. A minority of patients had NSTEMI: 34.0% (Impella), 25.2% (IABP), and 27.7% (vasopressors without MCS).
| Unmatched | Matched | |||||
|---|---|---|---|---|---|---|
| Characteristic | Impella (n = 14,069) | IABP (n = 1349) | SMD | Impella (n = 1320) | IABP (n = 1320) | SMD |
| Inpatient clinical diagnosis | ||||||
| AKI | 56.2 | 45.2 | 0.22 | 42.4 | 46.2 | 0.08 |
| Hypotension | 11.7 | 16.3 | 0.13 | 12.9 | 16.3 | 0.10 |
| Lactic acidosis | 35.2 | 25.9 | 0.2 | 29.5 | 26.5 | 0.07 |
| NSTEMI | 34 | 25.2 | 0.19 | 31.1 | 25.8 | 0.12 |
| Demographics | ||||||
| Age, median (IQR) | 66 (57–73) | 64 (57–76) | −0.015 | 65 (57–73) | 64 (56–76) | 0.009 |
| Male | 71.7 | 65.6 | 0.13 | 70.5 | 65.5 | 0.11 |
| Race | ||||||
| White | 69.9 | 71.5 | 0.04 | 72.7 | 71.6 | 0.10 |
| Black | 8.2 | 8.1 | 6.8 | 8 | ||
| Hispanic | 9.3 | 9.6 | 6.4 | 9.5 | ||
| Other | 12.6 | 10.7 | 14 | 11 | ||
| Medicare | 55.3 | 54.4 | 0.02 | 56.8 | 53.8 | 0.06 |
| Payer | ||||||
| Medicaid | 9.3 | 11.5 | 8.3 | 11.4 | ||
| Private | 27.2 | 25.9 | 26.5 | 26.5 | ||
| Self-pay | 5.4 | 3.3 | 5.3 | 3.4 | ||
| Other | 2.8 | 4.8 | 3 | 4.9 | ||
| Medical history | ||||||
| Diabetes | 12.5 | 12.6 | 0.003 | 14 | 12.5 | 0.04 |
| Hypertension | 24.7 | 37.8 | 0.29 | 35.6 | 36.4 | 0.02 |
| Hyperlipidemia | 49.8 | 53 | 0.06 | 53 | 52.7 | 0.008 |
| CAD | 84 | 83 | 0.03 | 88.3 | 83 | 0.15 |
| PCI | 11.4 | 13 | 0.05 | 14.8 | 12.9 | 0.05 |
| PAD | 5.9 | 7.4 | 0.06 | 5.7 | 7.2 | 0.06 |
| Obesity | 15.1 | 12.6 | 0.07 | 19.3 | 12.5 | 0.19 |
| CVA/TIA | 4.7 | 5.9 | 0.05 | 3 | 5.7 | 0.13 |
| Atrial fibrillation | 15.6 | 16.3 | 0.02 | 14.8 | 16.3 | 0.04 |
| Valvular disease | 14.9 | 13 | 0.06 | 13.6 | 12.9 | 0.02 |
| Smoking | 14.7 | 15.2 | 0.01 | 16.3 | 15.5 | 0.02 |
| COPD | 13.1 | 11.9 | 0.04 | 15.9 | 11.4 | 0.13 |
| Anemia | 2 | 2.6 | 0.04 | 1.1 | 2.7 | 0.11 |
| CKD | 24.5 | 16.7 | 0.2 | 19.7 | 17 | 0.07 |
| ESRD | 7.2 | 5.6 | 0.07 | 6.8 | 5.7 | 0.05 |
- Note: All values are listed as percentages, except for age which is listed in years.
- Abbreviations: AKI, acute kidney injury; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease stages 3–5; CVA, cerebrovascular accident; ESRD, end-stage renal disease; IABP, intra-aortic balloon pump; IQR, interquartile range; NSTEMI, non-ST elevation myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; SMD, standardized mean difference. Less than 0.25 indicates small difference between variables; TIA, transient ischemic attack.
| Unmatched | Matched | |||||
|---|---|---|---|---|---|---|
| Characteristic | Impella (n = 14,069) | Vaso (n = 2344) | SMD | Impella (n = 2269) | Vaso (n = 2269) | SMD |
| Inpatient clinical diagnosis | ||||||
| AKI | 56.2 | 57.6 | 0.03 | 57.9 | 58.1 | 0.004 |
| Hypotension | 11.7 | 15.1 | 0.1 | 12.3 | 15 | 0.08 |
| Lactic acidosis | 35.2 | 36.7 | 0.03 | 36.6 | 37 | 0.009 |
| NSTEMI | 34 | 27.7 | 0.14 | 28.2 | 27.3 | 0.02 |
| Demographics | ||||||
| Age, median (IQR) | 66 (57–73) | 66 (58–75) | −0.028 | 65 (57–72) | 66 (58–74) | 0.044 |
| Male | 71.7 | 67 | 0.1 | 71.4 | 67 | 0.1 |
| Race | ||||||
| White | 69.9 | 67.4 | 0.05 | 69.8 | 67 | 0.1 |
| Black | 8.2 | 7.9 | 7.5 | 7.7 | ||
| Hispanic | 9.3 | 8.5 | 9.9 | 8.8 | ||
| Other | 12.6 | 16.2 | 12.8 | 16.5 | ||
| Payer | ||||||
| Medicare | 55.3 | 58 | 0.05 | 54.2 | 57.7 | 0.07 |
| Medicaid | 9.3 | 9.6 | 10.1 | 9.7 | ||
| Private | 27.2 | 24.3 | 25.8 | 24.4 | ||
| Self-pay | 5.4 | 5.1 | 7.3 | 5.1 | ||
| Other | 2.8 | 3 | 2.6 | 3.1 | ||
| Medical history | ||||||
| Diabetes | 12.5 | 10 | 0.08 | 12.1 | 10.4 | 0.06 |
| Hypertension | 24.7 | 30.1 | 0.12 | 23.8 | 30.4 | 0.15 |
| Hyperlipidemia | 49.8 | 52.7 | 0.06 | 47.4 | 52.9 | 0.11 |
| CAD | 84 | 82.5 | 0.04 | 83.7 | 83.9 | 0.006 |
| PCI | 11.4 | 11.9 | 0.02 | 12.3 | 11.7 | 0.02 |
| PAD | 5.9 | 6.6 | 0.03 | 6. | 6.6 | 0.009 |
| Obesity | 15.1 | 18.3 | 0.09 | 17.4 | 17.8 | 0.01 |
| CVA/TIA | 4.7 | 7.5 | 0.11 | 5.5 | 7.5 | 0.08 |
| Atrial fibrillation | 15.6 | 20.3 | 0.12 | 17.2 | 20 | 0.07 |
| Valve disease | 14.9 | 15.4 | 0.01 | 15.2 | 14.8 | 0.01 |
| Smoking | 14.7 | 17.5 | 0.08 | 15.9 | 16.7 | 0.02 |
| COPD | 13.1 | 16.4 | 0.09 | 14.1 | 16.5 | 0.07 |
| Anemia | 2 | 2.1 | 0.007 | 1.8 | 2.2 | 0.03 |
| CKD | 24.5 | 27.1 | 0.06 | 25.8 | 26.7 | 0.02 |
| ESRD | 7.2 | 6.4 | 0.03 | 6. | 6.4 | 0.02 |
- Note: All values are listed as percentages, except for age which is listed in years.
- Abbreviations: AKI, acute kidney injury; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease stages 3–5; CVA, cerebrovascular accident; ESRD, end-stage renal disease; IABP, intra-aortic balloon pump; IQR, interquartile range; NSTEMI, non-ST elevation myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; SMD, standardized mean difference. Less than 0.25 indicates small difference between variables; TIA, transient ischemic attack; Vaso, vasopressors.
| Unmatched | Matched | |||||
|---|---|---|---|---|---|---|
| Characteristic | IABP (n = 1349) | Vaso (n = 2344) | SMD | IABP (n = 904) | Vaso (n = 904) | SMD |
| Inpatient clinical diagnosis | ||||||
| AKI | 45.2 | 57.6 | 0.25 | 43.1 | 51.9 | 0.18 |
| Hypotension | 16.3 | 15.1 | 0.03 | 17.7 | 11.6 | 0.17 |
| Lactic acidosis | 25.9 | 36.7 | 0.23 | 25.4 | 32 | 0.15 |
| NSTEMI | 25.2 | 27.7 | 0.06 | 24.3 | 28.2 | 0.09 |
| Demographics | ||||||
| Age, median (IQR) | 64 (57–76) | 66 (58–75) | −0.013 | 64 (56–77) | 67 (58–75) | 0.019 |
| Male | 65.6 | 67 | 0.03 | 63 | 64.1 | 0.02 |
| Race | ||||||
| White | 71.5 | 67.4 | 0.09 | 69.6 | 63 | 0.1 |
| Black | 8.1 | 7.9 | 7.2 | 7.7 | ||
| Hispanic | 9.6 | 8.5 | 10.5 | 7.7 | ||
| Other | 10.7 | 16.2 | 12.7 | 21.5 | ||
| Payer | ||||||
| Medicare | 54.4 | 58 | 0.07 | 52.5 | 56.4 | 0.08 |
| Medicaid | 11.5 | 9.6 | 12.7 | 8.3 | ||
| Private insurance | 25.9 | 24.3 | 28.7 | 26 | ||
| Self-pay | 3.3 | 5.1 | 2.8 | 6.6 | ||
| Other | 4.8 | 3 | 3.3 | 2.8 | ||
| Medical history | ||||||
| Diabetes | 12.6 | 10 | 0.08 | 14.4 | 12.7 | 0.05 |
| Hypertension | 37.8 | 30.1 | 0.16 | 37 | 32.6 | 0.09 |
| Hyperlipidemia | 53 | 52.7 | 0.006 | 54.7 | 60.2 | 0.11 |
| CAD | 83 | 82.5 | 0.01 | 86.2 | 89.5 | 0.1 |
| PCI | 13 | 11.9 | 0.03 | 12.7 | 12.7 | <0.001 |
| PAD | 7.4 | 6.6 | 0.03 | 7.7 | 6.6 | 0.04 |
| Obesity | 12.6 | 18.3 | 0.16 | 14.4 | 2 | 0.17 |
| CVA/TIA | 5.9 | 7.5 | 0.06 | 6.1 | 9.9 | 0.14 |
| Atrial fibrillation | 16.3 | 20.3 | 0.1 | 17. | 18.8 | 0.03 |
| Valve disease | 13 | 15.4 | 0.07 | 15.5 | 12.7 | 0.08 |
| Smoking | 15.2 | 17.5 | 0.06 | 15.5 | 17. | 0.04 |
| COPD | 11.9 | 16.4 | 0.13 | 13. | 17.1 | 0.11 |
| Anemia | 2.6 | 2.1 | 0.03 | 2.2 | 1. | 0.09 |
| CKD | 16.7 | 27.1 | 0.25 | 17.7 | 22.7 | 0.12 |
| ESRD | 5.6 | 6.4 | 0.04 | 6.1 | 5 | 0.05 |
- Note: All values are listed as percentages, except for age which is listed in years.
- Abbreviations: AKI, acute kidney injury; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease stages 3–5; CVA, cerebrovascular accident; ESRD, end-stage renal disease; IABP, intra-aortic balloon pump; IQR, interquartile range; NSTEMI, non-ST elevation myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; SMD, standardized mean difference. Less than 0.25 indicates small difference between variables; TIA, transient ischemic attack; Vaso, vasopressors.
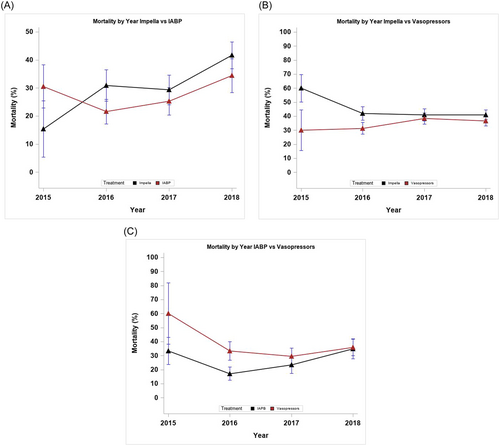
Figure 2 depicts mortality by year in each of the three study cohorts. Supporting Information: Figures S1 and S2 summarize mortality treatment contrasts by hospital size and year, as well as by region and year (Supporting Information: Figures S1A, S1B, S1C, S2A, S2B, S2C). No significant variation in the treatment effect on mortality was found by year, hospital size, or region (p > 0.05 for all interactions).
3.1 Impella versus IABP
The baseline characteristics of the unmatched Impella (n = 14,069) and IABP (n = 1349) groups are summarized in Table 1. After application of the propensity-matching algorithm, the SMD was ≤0.20 for all clinical variables. Impella patients had higher major bleeding than IABP patients (31.4% vs. 13.6%; odds ratio [OR]: 3.3; 95% confidence interval [CI]: 2.0–5.5; p < 0.001], with no significant difference in mortality (34.1% vs. 26.9%; OR: 1.5; 95% CI: 1.0–2.2; p = 0.06) (Table 4). Palliative care consult was higher in the Impella group (13.3% vs. 8.0%; OR: 1.9; 95% CI: 1.0–3.5; p = 0.04). The two groups had similar rates of cardiac arrest (p = 0.59). Total hospital charges were higher in the Impella group (p < 0.001). LOS was similar between both groups (p = 0.33).
| Outcome | Impella (n = 1320) | IABP (n = 1320) | OR (95% CI) | p Value |
|---|---|---|---|---|
| Mortality | 34.1 | 26.9 | 1.5 (1–2.2) | 0.06 |
| Major bleeding | 31.4 | 13.6 | 3.3 (2–5.5) | <0.001 |
| Limb ischemia | 0.4 | 0.8 | 0.5 (0–5.2) | 0.56 |
| Cardiac arrest | 12.1 | 14.8 | 0.9 (0.5–1.5) | 0.59 |
| Cardiac tamponade | 0.8 | 1.1 | 1.1 (0.2–5.5) | 0.89 |
| Sepsis | 11.7 | 12.9 | 0.9 (0.5–1.6) | 0.65 |
| Palliative care | 13.3 | 8 | 1.9 (1–3.5) | 0.04 |
| LOS (days) | ||||
| [0, 7] | 56.4 | 56.4 | 1.1 (0.9–1.4) | 0.33 |
| [8, 14] | 21.6 | 26.1 | ||
| [15, 21] | 13.6 | 10.6 | ||
| [22, +] | 8.3 | 6.8 | ||
| Total inpatient charges ($1000) | ||||
| [0, 101] | 5 | 28.2 | 2.8 (2.3–3.6) | <0.001 |
| (101, 167] | 14.1 | 25.6 | ||
| (167, 286] | 34.4 | 25.2 | ||
| (286, +] | 46.6 | 21 | ||
- Note: All values for Impella and IABP cohorts are listed as percentages, except for LOS which is listed in days and total charges which are listed in US dollars.
- Abbreviations: CI, confidence interval; IABP, inta-aortic balloon pump; LOS, length of stay; OR, odds ratio; USD, US dollars.
3.2 Impella versus vasopressors without MCS
The two groups were well-matched, with SMD less than ≤0.20 for all clinical variables (Table 2). Impella patients had higher mortality (42.3% vs. 35.7%; OR: 1.4 [1.1–1.9] p = 0.02) and major bleeding (33.9% vs. 22.7%; OR: 1.80 [1.30–2.40]; p < 0.001) compared to patients with vasopressors and no MCS (Table 5). The two groups had similar rates of cardiac arrest (p = 0.4) and palliative care consult (p = 0.24). Hospital charges were higher in the Impella group (p < 0.001). LOS was similar between the two groups (p = 0.73).
| Outcome | Impella (n = 2269) | Vasopressors (n = 2269) | OR (95% CI) | p Value |
|---|---|---|---|---|
| Mortality | 42.3 | 35.7 | 1.4 (1.1–1.9) | 0.02 |
| Major bleeding | 33.9 | 22.7 | 1.8 (1.3–2.4) | <0.001 |
| Limb ischemia | 1.8 | 0.7 | 2.9 (0.7–12.1) | 0.14 |
| Cardiac arrest | 14.1 | 15.2 | 0.9 (0.6–1.2) | 0.4 |
| Cardiac tamponade | 2.9 | 1.3 | 2.3 (0.9–6.2) | 0.1 |
| Sepsis | 16.5 | 19.6 | 0.7 (0.5–1.1) | 0.1 |
| Palliative care | 14.8 | 17.4 | 0.8 (0.6–1.2) | 0.24 |
| LOS (days) | ||||
| [0, 7] | 54.8 | 54.6 | 1 (0.9–1.2) | 0.73 |
| [8, 14] | 22.2 | 26.2 | ||
| [15, 21] | 12.3 | 9.5 | ||
| [22, +] | 10.6 | 9.7 | ||
| Total inpatient charges ($1000) | ||||
| [0, 101] | 3.1 | 22.7 | 2.8 (2.4–3.3) | <0.001 |
| (101, 167] | 11.7 | 28 | ||
| (167, 286] | 32.5 | 27.6 | ||
| (286, +] | 52.8 | 21.8 | ||
- Note: All values for Impella and IABP cohorts are listed as percentages, except for length of stay which is listed in days and total charges which are listed in US dollars.
- Abbreviations: CI, confidence interval; IABP, inta-aortic balloon pump; LOS, length of stay; OR, odds ratio; USD, US dollars.
3.3 IABP versus vasopressors without MCS
Baseline characteristics in the two groups were well-matched, with a SMD ≤ 0.20 for all clinical variables (Table 3). Palliative care consults (18.8% vs. 8.3%; p = 0.01) were higher in the vasopressors without MCS group (Table 6). The IABP and vasopressors without MCS groups were similar for all other outcomes, including mortality, major bleeding, cardiac arrest, LOS, and hospital charges (p > 0.05 for all).
| Outcome | IABP (n = 904) | Vasopressors (n = 904) | OR (95% CI) | p Value |
|---|---|---|---|---|
| Mortality | 25.4 | 33.7 | 0.8 (0.5–1.4) | 0.43 |
| Major bleeding | 12.2 | 18.8 | 0.6 (0.3–1.1) | 0.07 |
| Limb ischemia | 1.1 | 0.6 | 2.9 (0.3–29.6) | 0.37 |
| Cardiac arrest | 13.8 | 12.7 | 1.1 (0.6–2) | 0.77 |
| Cardiac tamponade | 1.1 | 0.6 | 1.6 (0.1–25.8) | 0.72 |
| Sepsis | 12.7 | 17.1 | 0.7 (0.4–1.4) | 0.33 |
| Palliative care | 8.3 | 18.8 | 0.4 (0.2–0.8) | 0.01 |
| LOS (days) | ||||
| [0, 7] | 53.6 | 54.1 | 1 (0.8–1.3) | 0.95 |
| [8, 14] | 30.9 | 29.3 | ||
| [15, 21] | 9.4 | 9.9 | ||
| [22, +] | 6.1 | 6.6 | ||
| Total inpatient charges ($1000) | ||||
| [0, 101] | 27.4 | 23.9 | 1 (0.8–1.2) | 0.71 |
| (101, 167] | 25.7 | 27.8 | ||
| (167, 286] | 24.6 | 22.8 | ||
| (286, +] | 22.3 | 25.6 | ||
- Note: All values for Impella and IABP cohorts are listed as percentages, except for length of stay which is listed in days and total charges which are listed in US dollars.
- Abbreviations: CI, confidence interval; IABP, inta-aortic balloon pump; LOS, length of stay; OR, odds ratio; USD, US dollars.
For NSTEMI, the significance of variation in the relationship between mortality and major bleeding with therapy (Impella, IABP, Vasopressors) was assessed with a statistical device by NSTEMI (Yes, No) interaction term in a fully adjusted logistic model on the same propensity-matched cohorts of the primary analyses. The results indicated no significant interactions, indicating that the odds ratios for mortality and bleeding did not vary significantly with the occurrence of NSTEMI when compared to the entire population of acute MI.
4 DISCUSSION
In this study using the NIS, in patients with AMI-CS undergoing PCI, Impella use was associated with higher major bleeding and no mortality benefit when compared to patients receiving IABP. Impella use was associated with higher mortality and major bleeding when compared to patients with vasopressors without MCS. IABP use was not associated with mortality benefit when compared to vasopressors without MCS. These results were obtained after adjusting for comorbidities and propensity-matching the cohorts of interest and is the largest such study to our knowledge to do so comparing MCS to vasopressor-only support. The key findings of our study are depicted in the Graphical Abstract. The most recent studies comparing Impella to IABP use in patients with AMI-CS either used data before 2015, did not specifically analyze a population of patients undergoing PCI, did not include lactic acidosis in the propensity-matching algorithm, and/or did not study a cohort with vasopressors without MCS.15, 17, 19, 20 A major difference in our study was that we addressed a cohort of patients who received only vasopressors and no other device therapy compared to those patients who received MCS (Impella or IABP) alone without vasopressor therapy. This was performed to best isolate the effects of each intervention. We used the most recent available NIS data with only ICD-10 codes and acute kidney injury, lactic acidosis, presence of NSTEMI (vs. STEMI), and hypotension as variables in the propensity-matching algorithm, reflecting an effort to optimize the degree of CS severity among all cohorts.
After an initial reduction in mortality with the advent of PCI, mortality rates in AMI-CS have plateaued.21 Despite the introduction of higher flow MCS devices and CS protocols, AMI-CS continues to be a clinical syndrome managed without rigorous, evidenced-based RCTs. Although the availability and ease with which MCS devices can now be placed has increased, there is no agreement on the specific situational use, patient selection, and management of MCS devices in this fragile patient population. To complicate matters, there is no universal definition of CS, and the definition has changed over time. The SHOCK Trial defined CS as systolic blood pressure (SBP) < 90 mmHg for >30 min or vasopressor support to maintain SBP > 90 mmHg and evidence of end-organ damage.22 The IABP-SHOCK II Trial defined CS as SBP < 90 mmHg for >30 min or needed infusion of catecholamines to maintain SBP > 90 mmHg, clinical signs of pulmonary congestion, or impaired end-organ). 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure define CS as “a syndrome due to primary cardiac dysfunction resulting in an inadequate cardiac output, comprising a life-threatening state of tissue hypoperfusion, which can result in multiorgan failure and death.” The guideline committee mandates the presence of clinical signs of hypoperfusion and biochemical markers of hypoperfusion (such as elevated serum creatinine, metabolic acidosis, and elevated serum lactate).3 In 2019, The Society for Cardiovascular Angiography and Interventions (SCAI) introduced a classification of CS, divided into five categories based on physical examination findings, biochemical markers, and hemodynamics.23 The current SCAI definition appears to be the most uniformly accepted definition of shock.
The most recent ESC Heart Failure guidelines recommend that in patients with CS, short-term MCS should be considered and IABP may be considered for circulatory support, an equivocal endorsement of a complex decision-making process.3 On one hand, the Impella leads to a clearly improved hemodynamic profile and stabilizes critically ill patients; on the other hand, there remains a lack of clear randomized evidence suggesting short-term or long-term benefits. Multiple studies examining Impella registry data have suggested that early initiation of MCS before PCI with Impella is associated with improved survival in the setting of AMI-CS.5-9 However, RCTs have not suggested that Impella use improves mortality in this population,13 although these trials have significant limitations in trial size and design.10 Previous studies of patients with AMI-CS using large databases have also suggested higher mortality in patients with Impella compared to IABP.15, 19, 20 The DanGer Shock study is a large RCT underway and is testing the hypothesis that MCS with the Impella device improves survival in patients with STEMI complicated by CS compared to conventional guideline-driven treatment.1 However, the results of this study are not yet available.
The findings of a 2017 meta-analysis of RCTs did not support the unselected use of MCS in patients with AMI-CS.24 In a 2019 retrospective analysis that used IABP-SHOCK II trial inclusion criteria, 237 patients treated with an Impella were matched to 237 IABP patients from the IABP-SHOCK II trial.25 Thirty-day mortality rates between Impella (48.5%) and IABP (46.3%) appeared similar. Our study also suggested significantly higher rates of major bleeding with Impella, which offers a possible explanation for higher mortality with Impella. Timing of device placement is not controlled for in these studies, which can be crucial. The likelihood of major bleeding as the cause of higher mortality in the Impella group in our study is further supported by the finding that IABP patients had similar major bleeding as well as similar mortality when compared to the vasopressors without the MCS group. Increased bleeding with the Impella device has been consistently demonstrated across multiple studies and is a logical finding, given the larger sheath size required for arterial access and the higher demand of anticoagulant administration.
Previous criticism of retrospective studies in patients with AMI-CS receiving MCS included that Impella patients likely represent a cohort that is more critically ill with more baseline comorbidities, which may lead to increased mortality. Using propensity-matching, including for the presence of acute kidney injury, lactic acidosis, presence of NSTEMI (vs. STEMI), and hypotension, the cohorts in our study were matched for comorbidities and acuity as best as possible. The patients in the vasopressor without MCS cohort when compared to the Impella cohort had a similar number of patients experiencing cardiac arrest and receiving palliative care consult which suggests similar distribution of critically ill patients. Although IABP was associated with lower cost than Impella, when compared to patients receiving vasopressor therapy with no MCS, there was no mortality benefit. The lack of mortality benefit for device therapy in general in our study underscores the theme in recent randomized and retrospective studies that despite higher cost and patients with similar acuity levels, there is no apparent mortality benefit for Impella and IABP patients, with a possible sign of harm. As our study population of AMI-CS undergoing PCI had overall high mortality despite device therapy, the concept of futility of care and the role of palliative care may need to be emphasized more in this population. Recent guidelines have highlighted the role of palliative care in patients with AMI-CS.3
Previous observational studies suggest an association between vasopressor use and mortality in patients with AMI-CS and Impella use has been suggested as a way to reduce mortality in such patients.26 These studies were non-randomized and implied that vasopressors may be associated with increased mortality but could not prove causation. A noteworthy finding in our study is that vasopressor use was not associated with increased mortality when compared to IABP use and was associated with reduced mortality compared to patients receiving Impella without vasopressors.
4.1 Limitations
This analysis should be interpreted within the context of certain limitations. The data are retrospective, and patients are not randomized; therefore, our results are subject to bias due to the inability to control all possible confounding variables. In the setting of a retrospective design, any statistical method, including propensity score matching, will not be adequate to address confounding by indication, for which randomized clinical trials offer the only option but are not available. However, the sample sizes were large enough to allow adjusted assessments of variation in the risk of several outcomes with each device, and vasopressor treatment and patients were randomly selected. SCAI shock stages for patients in our cohort could not be specified and such an analysis could better match baseline characteristics and assess for mortality benefit of Impella at each stage.27, 28 Additionally, the administrative data set design of NIS relies on user entry of ICD-10 coding which may be susceptible to variability in diagnosis. Interpretation of the definition of CS may vary between physicians entering ICD codes. ICD coding cannot differentiate between Impella RP for right ventricular CS and Impella CP versus Impella 5.5 for left ventricular CS. Due to these limitations, this study should not be viewed as a definitive referendum on the pitfalls of MCS but rather an impetus to be more judicious in its use and to better analyze this complex clinical problem with respect to device choice and timing of support.
5 CONCLUSION
This study suggests (within the limitations of the NIS database) higher mortality, major bleeding and in-hospital cost in patients with AMI-CS receiving Impella, as compared to patients receiving vasopressor support without MCS. Vasopressor use was not associated with increased mortality in our study compared to previous smaller observational studies. As previous observational studies suggest that timing of Impella placement may improve survival, it is possible that operators are not placing Impella in patients with AMI-CS before PCI and this is driving the lack of mortality benefit. Another possibility is that Impella devices are being placed in patients who have progressed too far in the clinical course of CS to realize benefit. Alternatively, it is possible that Impella use may be associated with harm due to increased bleeding. Our analysis highlights the need for large, randomized prospective trials to help clinicians select the optimal choice and optimal timing of therapy in patients with AMI-CS undergoing PCI.
ACKNOWLEDGMENTS
The University of Texas Health at San Antonio Department of Population Health Sciences for statistical contributions.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in HCUP NIS Database at https://hcup-us.ahrq.gov/tech_assist/centdist.jsp.