Transcatheter closure of large postinfarct ventricular septal defect: Initial results of prototype Occlutech® device including the first-in-human
Abstract
Background
Postinfarct ventricular septal rupture is a serious complication in delayed or failed reperfusion with a grim prognosis. The optimal timing and treatment option remain debatable in the absence of randomized controlled trials. Percutaneous device closure is a well-reported and less invasive treatment option but recent imaging studies indicate that majority of defects are too large to be adequately covered by the currently Conformite Europeenne and Food and Drug Administration approved occluder devices.
Methods
Six patients presented with large and complex postinfarct ventricular septal ruptures, considered unsuitable for the Amplatzer post-infarct ventricular septal defect Occluder, so were treated using the prototype Occlutech® 36 mm PI-VSD occluder, including the first-in-human use.
Results
The prototype device was successfully deployed in all patients with satisfactory immediate results and shunt reduction. Three patients (50%) in cardiogenic shock did not survive beyond discharge, of which two were complicated by device dislodgement or embolization.
Conclusions
Percutaneous closure of large postinfarct ventricular septal ruptures is possible using newer device with a wider coverage. Further device refinement is necessary to improve treatment outcomes.
Abbreviations
-
- CE Mark
-
- Conformitè Europëenne Mark
-
- CT
-
- computer tomography
-
- IABP
-
- intra-aortic balloon pump
-
- LAD
-
- left anterior descending
-
- MI
-
- myocardial infarction
-
- MRI
-
- magnetic resonance imaging
-
- PCI
-
- percutaneous coronary intervention
-
- PDA
-
- posterior descending artery
-
- RCA
-
- right coronary artery
-
- US-FDA
-
- United States Food and Drug Administration
1 INTRODUCTION
Postinfarct ventricular septal rupture has a very high mortality, so despite its low incidence in the postcoronary reperfusion era, it remains an important cause of death after myocardial infarction (MI). Contemporary data estimates an incidence between 0.31% and 0.71% in all acute MIs.1 Delay or failure in instituting effective reperfusion is a key contributing factor leading to the development of septal rupture and frequently resulting in cardiogenic shock due to left-to-right ventricular shunt. This causes an in-hospital mortality as high as 87% despite the best possible treatments.2, 3 The optimal mode of intervention continues to be a subject of debate as open surgery, which in theory confers a definitive mechanical solution, is often hampered by comorbidities, poor tissue quality in the infarcted area and acute multiorgan dysfunction.4 Percutaneous device closure offers a less invasive alternative with minimal haemodynamic disruption to achieve shunt reduction and circulatory stabilization.
However, septal rupture due to myocardial necrosis is often unpredictable in terms of its location and dimensions. Recent computed tomography (CT) derived data from 32 of our previous patients published by Hamilton et al. demonstrated an average defect size of 26.5 ± 11.5 mm with mean eccentricity index of 1.7 ± 0.5.5 This data suggests that a great majority of defects presenting to a cardiac department were too large to be adequately covered by the currently available devices, for instance the largest approved Amplatzer® PI-VSD Occluder (Abbott Vascular) has a waist diameter up to 24 mm. The lack of larger sized devices explains device-related complications such as embolization and residual leak, even after successful deployment, leading to suboptimal outcomes.6 One would expect larger devices to be easier to correctly position and should have lower rates of embolization and residual leaks, even accounting for the possibility that the freshly infarcted tissues will undergo further necrosis and thus enlargement of the defect.
We report our initial experience in 6 patients, including the first use in a human, who had large and complex post-infarct ventricular septal defects which were treated with the Occlutech® 36 mm PI-VSD Occluder (Occlutech GmBH), a non- Conformite Europeenne (CE) marked, non-United States Food and Drug Administration (US-FDA) approved prototype used on a compassionate basis.
2 METHODS
2.1 The patients
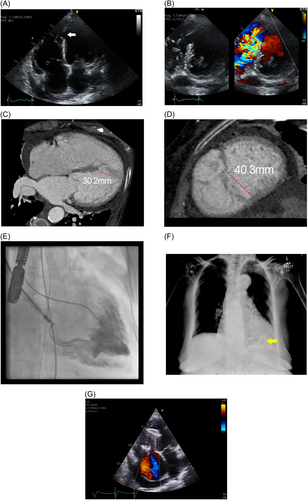
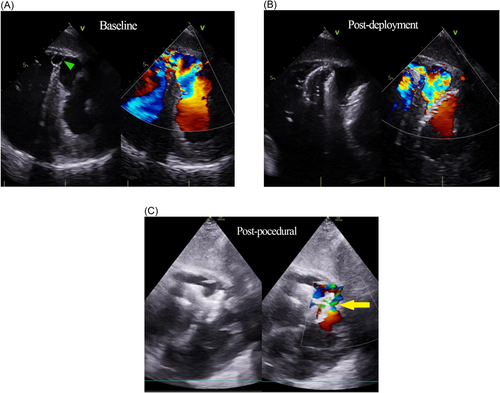
The initial first-in-human implantation was performed on March 16, 2018 at the Bristol Heart Institute, United Kingdom (Figure 1). Subsequently, the device was implanted in another five patients until December 2020, age range was 50−77 years old. Three patients were treated emergently in cardiogenic shock, 2 with insidious heart failure and 1 diagnosed incidentally with noncardiac symptoms, but volume loaded ventricles. Timing of MI or failed reperfusion was known in four patients. One patient received salvage percutaneous coronary intervention to the culprit vessel during the acute presentation. Diagnoses were made by transthoracic echocardiography which demonstrated presumably new ventricular left-to-right shunts, associated right ventricular volume overload and tricuspid regurgitation. We have not reported the left ventricular ejection fractions, as volumetric assessments can be unreliable, given that the systemic chambers were often decompressed due to offloading of volume into the low-pressure right ventricle. An intra-aortic balloon pump was inserted in all patients either for circulatory support in shock or perioperatively before the procedures. Additionally, an Impella ventricular assist device (Abiomed Inc) was used in 1 patient. Electrocardiography-gated cardiac CT scans were used for defect delineation and characterization. All patients were reviewed and discussed in the multidisciplinary heart team meetings to evaluate the surgical risk and determine the best treatment option. Surgical consultations were made for three patients who deteriorated after initially successful procedures, complicated by device embolization, migration and nosocomial infection, and died shortly after due to intractable shock. No surgical intervention was offered as it was deemed too high risk and futile.
2.2 The device and delivery system
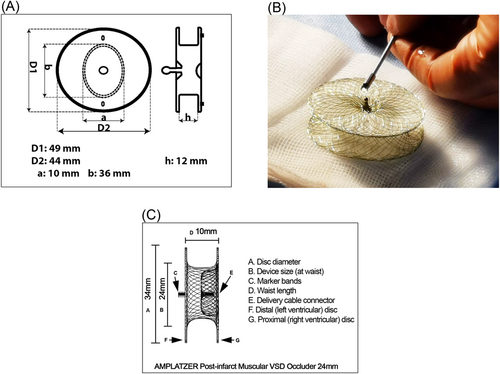
Occlutech® PI-VSD Occluder is a prototype dedicated for transcatheter closure of postinfarct ventricular septal defect. The device has a symmetrical double-disc nitinol frame with a broad waist to accommodate the surrounding muscular rim. The braiding technology allows all nitinol strands to converge into a single-sided hub, which attaches to the delivery system via a ball-and-socket mechanism for flexibility. The distal component devoid of any central attachment structure possibly being less traumatic, and a left-sided disc that has less foreign material within the systemic circulation. Two orientation indicators mark the longitudinal axis of the device, but with the current delivery systems and closure technique, the ability to rotate the device to an optimal orientation during deployment is limited. The polyethylene terephthalate patches sewn within the disc frames form the sealing zone across the defect once it is fully expanded.
The 36 mm variant is the largest of the prototype devices. The waist is oval in shape measuring 10 x 36 mm with two corresponding discs, each of 44 x 49 mm in size, 12 mm apart; it does not bear European CE mark or US-FDA approval for clinical use (Figure 2).
This device was chosen according to the defect measurements from the CT data, which other alternatives were too small or deemed inadequate to provide a sufficient seal and stability. Approval from United Kingdom Medicines and Healthcare products Regulatory Agency (MHRA) was obtained individually for each patient before clinical use.
2.3 The technique
All procedures were performed under general anaesthesia. Vascular access from the femoral artery and right internal jugular vein were obtained under ultrasound guidance. The interventricular defect was crossed from the femoral artery access, via the aortic valve and then from the left ventricle using 0.035″ hydrophilic coated guidewire with the support of either a JR-4 or AL-1 catheter. Once across the defect, the distal end of the wire was snared by a 30 mm Amplatz Goose Neck Snare (Medtronic) in the pulmonary artery, and was then externalized at the jugular vein. Close attention was paid to the tricuspid valve leaflet movement after the formation of arterio-venous loop, as new-onset regurgitation will indicate chordae entanglement. Next, the support catheter was advanced to facilitate wire exchange to the coreless Amplatzer Noodlewire guidewire (Abbott Vascular). From the right internal jugular vein, an Occlutech 14-Fr delivery sheath was inserted and pushed gently across the septal defect, guided and pulled up away from the apex, by the wire loop. Subsequently, an Occclutech 36 mm PI-VSD occluder was advanced and the left disc deployed in the left ventricle under trans-esophageal echocardiography and fluoroscopy guidance. The device placement was scrutinized to ensure that the left disc was aligned to the left ventricular septal border for maximal coverage and the waist well-compressed within the defect body. The morphology and function of the mitral valve were observed throughout for possible chordae entanglement. Complete right disc expansion was often unachievable due to entrapment by the surrounding trabeculae or intramyocardial dissection flap. Lastly, device stability and reduction in shunt volume were demonstrated before final release.
3 RESULTS
| Patient | Age/sex | Relevant history | Defect Size/Location | Investigations and interventions | Comments |
|---|---|---|---|---|---|
| A | 87/Female | Presented with subacute heart failure. History of pulmonary embolism on anticoagulation. No recallable cardiac event. | 30 x 40 mm/Basal Inferior | Unobstructive coronary arteries, myocardial infarction possibly due to past thrombotic or embolic event. | Successful deployment. Marked clinical improvement of heart failure. Alive at 3 years follow-up. |
| B | 54/Male | Schizophrenia. Recent missed inferior MI but unsure of exact date. Presented with acute heart failure with multiorgan dysfunction. | 25 x 36 mm/Mid Inferior | Triple vessel disease with occluded large posterior descending artery (PDA), no intervention. | Successful deployment. Residual shunt caused haemolysis (haematuria) requiring transfusion, resolved within 2 weeks. Alive at 2 years follow-up. |
| C | 77/Male | Late presentation inferior MI with cardiogenic shock 2 weeks prior. PCI to right coronary artery (RCA) but incomplete reperfusion. Persistent shock with multiorgan dysfunction | 23 x 27 mm/Mid Inferior | Single vessel RCA occlusion, treated with 2 drug eluting stents | Successful initial deployment but device embolized to right ventricle. Died 4 days later due to intractable shock. |
| D | 62/Male | Prior anterior MI 6 weeks ago with primary PCI to LAD but poor distal flow. Incidental finding of new heart murmur. Clinically asymptomatic. | 14 x 20 mm/Basal Anterior | Full thickness left anterior descending artery (LAD) territory infarct with microvascular obstruction on MRI. (Ejection Fraction 44%) | Successful deployment. Small residual leak on follow-up. Alive and well at 2 years follow-up. |
| E | 64/Male | Delayed presentation of inferior MI. Cardiogenic shock with acute kidney injury. | 19 x 20 mm/Basal Inferior | Triple vessel disease with left main stem involvement, no intervention | Successful deployment. Initial clinical improvement but died 9 days later due to nosocomial pneumonia. |
| F | 50/Male | Delayed presentation of Inferior MI in profound cardiogenic shock requiring Impella® and IABP support. | 24 x 33 mm/Basal Inferior | Acute occlusion of RCA with LAD chronic total occlusion, salvage PCI to RCA and LAD arteries. | Successful deployment but left disc dislodged postprocedure causing worsening shunt. Died 2 days later due to intractable shock. |
- Abbreviations: IABP, intra-aortic balloon pump; PCI, percutaneous coronary intervention.
4 DISCUSSION
This paper describes the technical feasibility of using the prototype Occlutech PI-VSD Occluder in a group of patients with large and complex post-infarct ventricular septal ruptures. Unlike most reported series, CT scan was used as the primary imaging modality for procedural planning. We previously reported the use of CT to delineate margin-to-margin defect diameters, as well as for anatomical characterization.5 Accurate device sizing in post-infarct VSR is challenging and universal consensus among interventionists is lacking. To achieve adequate device coverage and stable anchoring, oversizing is widely advocated, either to double the device size against the defect diameter, or at least 10 mm larger.7 Therefore, the development of larger occluder devices is in practice reasonable and well-justified.
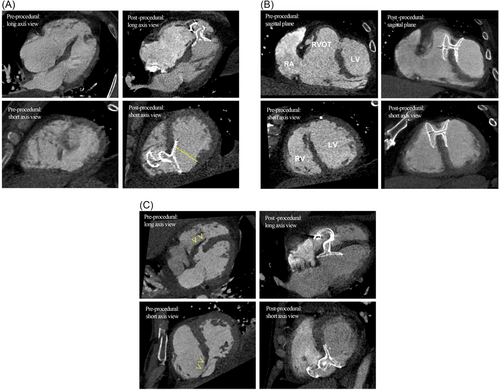
One may argue if the already-approved devices could have been used to close smaller defects in our series, but our experience shows that in complex anatomies, for instance in the presence of long dissection tunnel and limited anchoring rims, anatomical distortion and configurational changes may result in inadequate sealing. (See CT panels for patient D and E) (Figure 3). This observation possibly explains why complete sealing is not always achievable in real life, if sizing was oversimplified, merely by matching the device's waist width to the defect diameter. It is also apparent on the CT images that postinfarct VSR are almost always irregular, complex and never truly round in shape. Our previously CT data suggested that the 34 mm disc of the largest Amplatzer PI-VSD device could only provide left-sided coverage in 75% of the cases, 50% if measured by its 24 mm waist.5 Alternatively, the Amplatzer atrial septal occluders can in theory confer wider coverage, up to 40 mm, and the experience has been reported in isolated case reports with good procedural success.8 However, its clinical usage remains outside its design indication. It was shown in a major review series that Amplatzer Septal Occluders was reportedly the least preferred option in treating post-infarct VSR, as compared to other more robustly constructed alternative, such as the dedicated P.I muscular VSD occluders or even the regular muscular VSD occluder.9 Other authors also expressed concerns over its softer construct and higher permeability to withstand high interventricular gradient.10
The mortality rate associated with VSR closure varies widely. In a meta-analysis comparing the outcomes of medical therapy, surgical and transcatheter closure, overall 30-day mortality was reported at 51%, while the transcatheter subgroup had a mean of 33% with a wide standard deviation of 24%.11 No superiority was shown between surgical and transcatheter approaches, but medical therapy was consistently linked to poorer outcome. However, technical success does not necessarily translate into survival. Our series has demonstrated successful device placement in all patients, but three patients (50%) who presented with cardiogenic shock failed to survive beyond discharge (Figure 4). This observation is consistent with most contemporary studies, highlighting the significance of cardiogenic shock on short term prognosis.12 Multicentre observational data from the United Kingdom (Calvert et al.) indicated that, despite a high implant success rate of 89%, only 58% survived until discharge, and only 23% had complete shunt reduction.13 Among the many negative predictive factors such as older age, female sex, baseline haemodynamic instability and the absence of revascularization therapy, larger defect size appeared to be an important contributor leading to higher mortality. In contrast, immediate shunt reduction was associated with survival benefit, implicating the importance of achieving a good seal, even in large defects.
New occluder devices offering wider coverage and better conformability appear to be a clinical necessity. The large 36 mm Occlutech prototype device comes with a 44 x 49 mm left-sided disc allowing a wider reach, and possibly better deployment stability and accuracy. The oval shape waist provides some centering of the device within the defect, but changing the orientation during deployment is not always achievable, leaving the design intention questionable. Often, the right disc failed to fully expand due to constraint within the intramyocardial dissection flap or interference from the surrounding structures, giving rise to the appearance of “cobra” deformity. The mechanism of this phenomenon is however different from those reported in atrial septal closures where excessive twisting and rotation of the nitinol wires during delivery resulting in device deformation.14, 15 The long-term clinical implication is unknown in the absence of established data, but in our context it does provide an unintended benefit, acutely providing added stability to the device postimplantation. We decided to accept the final outcomes due to clinical reasons, and effective technical counter-measurements seemed limited. This dilemma was also echoed by colleagues (Demir et al.) who recently published a case report, describing similar finding using a 17 mm Amplatzer septal occluder.16 We propose that future device design should have a more downsized right-sided disc and a versatile delivery system to improve sealing, yet minimizing interactions with the surrounding structures.
5 CONCLUSION
Cumulative evidence and clinical experience have proven the feasibility and value of percutaneous closure of post-infarct ventricular septal rupture, even in large and structurally complex lesions. Improvements in occluder design, guided by imaging studies, should provide optimal haemodynamic outcomes and hopefully better survival.
ACKNOWLEDGMENTS
We are very grateful to Mr Shakeel Osman and colleagues at Occlutech UK for their support to treat these patients and for their assistance in obtaining Medicines and Health products Regulatory Agency (MHRA) regulatory compassionate approval.
CONFLICT OF INTEREST STATEMENT
Mark Turner was a Consultant for Occlutech and Medtentia and a Consultant and Proctor for Abbott, Medtronic, and Edwards Lifesciences. The remaining authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.