PERFORMANCE 1 study: Novel carotid stent system with integrated post-dilation balloon and embolic protection device
ClinicalTrials.gov Identifier: NCT04142541
Abstract
Objectives
The PERFORMANCE I study was designed to evaluate the safety and feasibility of the Neuroguard IEP® System, a novel carotid stent system with an integrated embolic filter and post-dilatation balloon, to treat clinically significant carotid artery stenosis.
Background
The risk of major adverse events during carotid artery stenting is comparable to carotid endarterectomy, however, the risk of minor stroke remains higher with stenting.
Methods
In total, 67 patients undergoing carotid artery stenting were enrolled at nine centers in Europe. Follow-up assessments included neurological exams, duplex ultrasound, 12-lead electrocardiogram, and cardiac enzyme analysis. The primary endpoint was the 30-day composite rate of stroke, death, and myocardial infarctions versus a prespecified performance goal. Secondary endpoints included procedure success, device success, and target lesion revascularization.
Results
The study population was predominantly male (74.6%) with a mean age of 69.3 ± 8.9 years and 67% of subjects met at least one criterion placing them at an elevated risk for adverse events following carotid endarterectomy. All patients were treated successfully with the study device. There were no deaths or strokes within 30 days of the index procedure. One subject (1.5%) experienced a non-ST elevation myocardial infarction at day 17. The primary endpoint was met with a 30-day major adverse events rate of 1.5% (1/67). Through 12-month follow-up, there were no strokes, neurological deaths, target lesion revascularizations, or instances of in-stent-restenosis.
Conclusions
Results from this study demonstrate the Neuroguard IEP system is safe and feasible with a stroke/death rate of 0% at 30 days. A large pivotal study is currently underway.
1 INTRODUCTION
Over the past two decades, carotid artery stenting (CAS) for the prevention of stroke in patients with symptomatic and/or asymptomatic carotid disease has evolved from a technically feasible approach in high surgical risk patients to a legitimate alternative to carotid endarterectomy in standard risk patients.1, 2 However, despite the use of embolic protection devices (EPD), minor stroke at the time of carotid stenting remains a problem. Most randomized trials comparing CAS to carotid endarterectomy (CEA) have shown no significant differences between the two modalities in the overall risk of death, stroke, and myocardial infarction (MI) at 30 days, but the risk of minor stroke has been consistently higher with CAS.1, 3, 4 During CAS performed using filter-based EPDs, monitoring of the middle cerebral artery by transcranial Doppler has shown that embolic particles reach the middle cerebral artery despite the use of embolic protection.5 While embolic signals are detected during all phases of the procedure, the majority of emboli occur during self-expanding stent placement and when the stent is dilated by an angioplasty balloon at high pressure, or post-dilation.6-9 Such emboli result in new ischemic lesions on diffusion-weighted magnetic resonance imaging (DW-MRI), and embolization, specifically during the post-dilation phase, has been shown to be an independent predictor of adverse outcomes.6, 7, 10
Although currently available EPDs are beneficial in preventing large embolic particles from reaching the brain, they are not completely effective in preventing all embolic particles from entering the intracranial circulation.11, 12 Such microemboli that reach the brain, despite the use of EPDs, may be responsible for the persistent higher risk of minor stroke during CAS as compared to CEA and may even be responsible for long term cognitive decline.13 Strategies to reduce the embolic risk during CAS have shown a reduction in the risk of minor stroke.2, 14, 15 The Neuroguard IEP® 3-in-1 Carotid Stent and Post-Dilation Balloon System with Integrated Embolic Protection (Neuroguard IEP System [Contego Medical, Inc]) (Figure 1) is a 6F system consisting of a nitinol self-expanding stent loaded on to a catheter, which consists of a post-dilation balloon and an integrated embolic protection filter with 40 µm pores designed to capture microemboli produced during the stenting procedure. It was designed to not only reduce the number of steps in the CAS procedure but was also designed to increase the degree and efficiency of embolic protection during the stenting and post-dilation phases, thus reducing the number of embolic particles reaching the brain. The PERFORMANCE I Study (Protection against Emboli using a 3-in-1 delivery system comprising an integRated Filter, nORMal Angioplasty balloon and Novel Carotid stEnt) was a prospective study designed to evaluate the safety and feasibility of the Neuroguard IEP System when used in patients with clinically significant carotid artery stenosis (symptomatic with ≥50% stenosis or asymptomatic with ≥80% stenosis) requiring revascularization.

2 METHODS
2.1 Study design and oversight
The PERFORMANCE I Study was a prospective, international, multi-center, single-arm, open label study that included nine European centers. The study was conducted in accordance with the Declaration of Helsinki and ISO 14155:2011 and approved by local competent authorities/ethics committees at each site. Patients provided written informed consent before any study-related procedures. An independent clinical research organization (genae nv, Belgium) managed regulatory submissions and electronic data collection. Data were 100% source verified. An independent clinical events committee reviewed reported events and made the final decisions on major endpoints including death, stroke, (including transient ischemic attacks [TIAs]) restenosis and device failures. An independent angiographic core laboratory (Yale Cardiovascular Research Group) analyzed angiograms. The study was prospectively registered at clinicaltrials. gov (NCT04142541).
Patients were eligible for the study if they were symptomatic (TIA or stroke within 180 days) with ≥50% carotid stenosis (as determined by angiography using NASCET methodology) or asymptomatic with ≥80% carotid stenosis. Key selection criteria are shown in Table 1.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
2.2 Study device and procedure
The Neuroguard system (Figure 1) is designed to deliver a self-expanding stent to the carotid arteries using a rapid exchange delivery system while simultaneously providing embolic protection without additional catheter or device exchanges. It is a 6 Fr device compatible with any commercially available 0.014″ guide wire. It consists of a multi-lumen shaft with an inflatable semi-compliant angioplasty balloon at the distal end and a handle on the proximal end. Distal to the angioplasty balloon is an integrated filter in the baseline-collapsed state. A nitinol self-expanding stent is preloaded on top of the angioplasty balloon. The stent has a closed cell design with an asymmetrical hourglass configuration with flares on either end and is designed to balance flexibility and radial strength. Both the stent and filter are covered by an outer sheath.
Before the index procedure, patients were pretreated with clopidogrel 75 mg (or equivalent) and aspirin ≥75 mg daily for 7 days. Dual antiplatelet therapy (DAPT) was required to continue for at least 4 weeks postprocedure and per investigator discretion beyond that timeframe. Angiography confirmed all angiographic inclusion criteria were met and none of the exclusion criteria were met. For patients who did not meet all selection criteria, commercially available devices were used and those patients were considered screen failures.
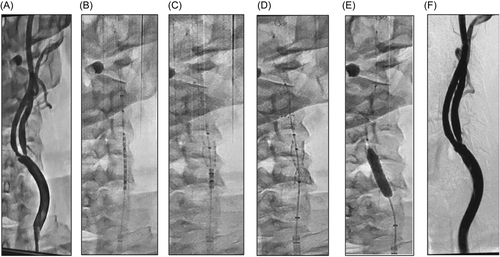
After enrollment was confirmed, anticoagulation was administered to achieve and maintain an activated clotting time (ACT) of at least 250 seconds. Once an ACT of ≥250 seconds was confirmed and a wire had crossed the target lesion, a commercially available primary embolic protection device was placed in the distal cervical/pre-petrous segment of the target internal carotid artery (ICA). Any commercially available filter-based EPD could be used at the physician's discretion. Pre-dilation was required if the lesion was ≥90% stenosed or if the operator believed direct stenting was not feasible, otherwise “direct stenting” was permitted. If pre-dilatation was necessary, the primary filter was required to be in place before that step. The Neuroguard system was then advanced over the wire of the primary EPD and positioned within the carotid artery such that the filter was located immediately beyond the lesion. When the stent was to be covering the lesion with a minimum of 0.5 cm extending distal and proximal to the target lesion, the constraining sheath was retracted using a thumb wheel on the proximal handle to expose the filter. A marker on the sheath indicated when the filter had been fully uncovered. The filter was then deployed by turning the filter activation knob on the handle counter-clockwise. At this point, the filter diameter and wall apposition were fluoroscopically confirmed. The constraining sheath was then retracted further to expose the stent, allowing it to expand and deploy within the lesion. Without adjusting the position of the catheter, the underlying balloon was then inflated to post-dilate the stent. The balloon was then deflated, and the filter was collapsed by clockwise rotation of the filter activation knob, thus securing captured embolic debris within the filter membrane. The delivery system and the primary filter were then sequentially removed from the patient. The angiographic images in Figure 2 illustrate the sequence of events during the treatment of a stenotic ICA lesion.
2.3 Study assessments and endpoints
All patients underwent clinical and neurological examinations before and after the procedure and again at their 30-day follow-up visit. These examinations included National Institutes of Health Stroke Scale (NIHSS), modified Rankin Scale, 12-lead electrocardiogram (ECG), cardiac enzyme analysis, a physical exam and an adverse event assessment. Follow-up at 6 and 12 months included NIHSS, carotid duplex ultrasound, physical exam and adverse event assessment. The NIHSS assessments were performed by certified and independent clinicians.
The study's primary endpoint was the 30-day rate of major adverse events (MAE), defined as the cumulative incidence of any periprocedural (≤30 days postprocedure) death, stroke or MI. Major stroke defined as a new focal ischemic neurological deficit of abrupt onset which is present after 7 days and results in a >4 point increase in the NIHSS score compared to baseline. Minor stroke was defined as a new focal ischemic neurological deficit of abrupt onset lasting >24 h and increases the NIHSS by <3 points at 7 days. MI was defined as creatine kinase-MB level that is 2X the upper limit of normal or higher as per the site's local laboratory, in addition to either chest pain or symptoms consistent with ischemia or ECG evidence of ischemia, including new ST segment depression or elevation of more than 1 mm in two or more contiguous leads.
The secondary endpoints included: (1) procedure success; defined as successful Neuroguard carotid stent implantation, or ≤50% residual angiographic stenosis, as determined by the angiographic core laboratory at the target lesion and no device malfunctions; (2) device technical success; defined as successful deployment of the stent in the planned targeted treatment location with a residual diameter stenosis ≤50% immediately after pos-dilatation per angiographic core laboratory, successful delivery and deployment of the Neuroguard filter beyond the target lesion and successfully retrieved after completion of the stent placement, successful post-dilation of the Neuroguard stent with the integrated angioplasty balloon, and successful removal of the delivery system; (3) late ipsilateral stroke (days 31–365); (4) target lesion revascularization defined as any revascularization procedure of the original treatment site associated with narrowing of >80% within 12 months of the index-procedure; and (5) in-stent restenosis (>70% restenosis per repeat angiography or ultrasound).
2.4 Statistical analysis
The study was designed to test the null hypothesis that the primary endpoint (30-day MAE rate) using the Neuroguard system is equal to or higher than the performance goal derived from historical controls. Based on a meta-analysis fixed effect model, the 30-day expected MAE rate was estimated at 5.4%. An adjustment of the 30-day expected MAE rate was calculated to specify that the highest composite 30-day MAE rate for this study is still within an acceptable range of the outcomes of the published studies. An analysis of the distribution of the upper 95% CI bounds for the historical 30-day MAE rates was used to determine an acceptable adjustment. A margin of 5.4% above the 30-day expected MAE rate estimate was selected to establish the performance goal. That is, the upper boundary of the 95% CI around an observed 30-day MAE rate in the PEFORMANCE I Study would be less than a performance goal of 8.1%. Secondary endpoints were evaluated using descriptive statistics only, no formal hypotheses were tested. Data were analyzed using SAS version 9.4 (SAS Institute).
2.5 Filter content analysis
A histomorphometric analysis was performed on a subset of the Neuroguard and primary filter contents immediately following retrieval from the patient. Fixed samples were sent to independent pathology laboratory (Sedgewick Imaging and Analysis). Morphometric analyses of the size and number of particles were performed using a 4800dpi Epson scanner with 20 µm detection capability. Images were postprocessed using incremental high pass filter radii in Fourier space followed by binarization of particles. Particle counts were determined, recorded, and categorized according length into the following ranges: 40–100, 101–200, and 201–400 µm.
3 RESULTS
3.1 Angiographic and procedural results
In total, 67 patients were enrolled February 2018 through January 2019 at nine centers in Europe. All patients were treated with the study device and no patient was lost to follow-up. Patient demographics are shown in Table 2.
| Demographics | N = 67 |
|---|---|
| Age (years) | 69.3 ± 8.9 |
| >70 | 28 (41.8) |
| Male | 50 (74.6) |
| Symptomatic (TIA or stroke within 180 days) | 11 (16.4) |
| Diabetes mellitus | 23 (34.3) |
| Hyperlipidemia | 55 (82.1) |
| Hypertension | 63 (94.0) |
| Current smoker | 24 (35.8) |
| History of carotid endarterectomy | 1 (1.5) |
| History of percutaneous carotid intervention, contralateral | 7 (10.4) |
| History of coronary artery disease | 33 (49.3) |
| Previous myocardial infarction | 10 (14.9) |
| History of coronary artery bypass graft | 6 (9.0) |
| History of percutaneous coronary intervention | 19 (28.4) |
| History of TIA or stroke | 13 (19.4) |
- Note: Values are mean ± standard deviation or n (%).
- Abbreviation: TIA, transient ischemic attack.
Lesion characteristics are shown in Table 3. The majority of lesions (76.1%) were located in the ICA; the mean target lesion length was 17.8 mm ± 7.6; the mean percent target lesion stenosis was 81.4% ± 10.8%.
| Target lesion characteristics | N = 67 |
|---|---|
| Lesion location | |
| Left ICA | 26 (38.8) |
| Right ICA | 25 (37.3) |
| Left ICA + bifurcation | 10 (14.9) |
| Right ICA + bifurcation | 6 (9.0) |
| Eccentric | 25 (37.3) |
| Concentric | 40 (59.7) |
| Calcification | 11 (16.4) |
| Ulceration | 8 (11.9) |
| Target lesion length (mm)a | 17.8 ± 7.6 |
| Target lesion stenosis (%)a | 81.4 ±10.8 |
| Target vessel reference diameter (mm) | 5.4 ± 0.8 |
| Target lesion diameter at most severe stenosis (mm) | 1.7 ± 1.1 |
| Procedural characteristics | |
| Access site | |
| Right femoral | 47 (70.1) |
| Left femoral | 2 (3.0) |
| Right radial | 18 (26.9) |
| Left radial | 0 (0) |
| Pre-dilation before Neuroguard system placement | 19 (28.4) |
| Primary embolic protection device type | |
| Distal | 66 (98.5) |
| Proximalb | 1 (1.5) |
| Primary embolic protection device | |
| Emboshield ® NAV 6 | 30 (44.8) |
| FilterWire EZ™ | 12 (17.9) |
| Mo.Ma Ultra® | 1 (1.49) |
| SpiderFX® | 24 (35.8) |
| Neuroguard filter successfully deployed | 67 (100) |
| Neuroguard stent successfully deployed at target lesion | 67 (100) |
| Post-dilation with integrated Neuroguard angioplasty balloon | 67 (100) |
| Dissection | 0 (0) |
| Filter landing zone vessel diameter (mm) | 4.8 ± 1.2 |
| Post stenting and post-dilation final stenosis (%) | 8.4 ± 12.5 |
| Angiographic evidence of distal embolization to cerebral arteryb | 0 (0) |
| Dissection | 0 (0) |
- Note: Values are mean ± standard deviation or n (%).
- Abbreviation: ICA, internal carotid artery.
- a Per angiographic core lab assessment.
- b Proximal protection was not permitted under the study protocol, this was captured as a protocol deviation.
All patients were treated successfully with the study device, achieving a residual angiographic stenosis of ≤50% as determined by the angiographic core laboratory without any device malfunctions. There were no instances of spasm, distal embolization, perforation or dissection as assessed by the core laboratory poststent deployment. In total, 18 (26.9%) patients were treated via radial approach, and all were successful. Proximal balloon occlusion was used in one patient. Sixty-six subjects were treated with a single Neuroguard stent. One patient received a Neuroguard stent and a second overlapping commercial stent without incident due to visual underestimation of the lesion length. Procedure and technical success endpoints were achieved in 100% of patients. The procedural characteristics are presented in Table 3.
3.2 Acute outcomes
The primary endpoint of periprocedural MAE was 1.5% (1/67). The upper 95% confidence limit was 7.7%, lower than the performance goal of 8.1% and indicating the primary endpoint was met. There were no neurological deaths or strokes between 30 days and 1 year of the index procedure. One subject (1.5%) experienced a non-ST elevation MI at Day 17 postprocedure, requiring percutaneous coronary intervention. This patient was not compliant with standard of care and the protocol requirement to remain on DAPT for 30 days following the procedure and had pre-existing coronary artery disease. All other patients were compliant with DAPT regimen through 30 days. Clinical events committee adjudication determined this event was not related to the device.
3.3 Long-term outcomes
Long-term outcomes were assessed through 12 months. All 67 patients were evaluated at the 30-day and 6-month follow-up. One subject died at month 7 of non-neurological causes. Of the remaining 66 patients, all completed the 12-month follow-up visit. There were no minor or major ipsilateral strokes and no neurological deaths through follow-up. There have been no reported TIAs or strokes for the subjects treated with the Neuroguard stent, none of the subjects have undergone a repeat intervention and 100% (66/66) of lesions were patent as determined by Duplex ultrasound at 12 months. There was no in-stent restenosis at 1 year. Endpoint results are reported in Table 4.
| Primary endpoint | |
| 30-day major adverse events | 1 (1.49%) (95% CI: 0.1% – 7.7%) |
| 30-day stroke | 0 (0%) |
| 30-day death | 0 (0%) |
| 30-day MI | 1 (1.49%) |
| Acute secondary endpoints | |
| Procedure success | 67 (100) |
| Successful neuroguard stent implantation | 67 (100) |
| ≤50% residual angiographic stenosisa | 67 (100) |
| No procedural device malfunctions | 67 (100) |
| Technical success | 67 (100) |
| Long-term secondary endpoints (through 12 months) | |
| Late ipsilateral stroke (day 31 through 365) | 0 (0) |
| Major | 0 (0) |
| Minor | 0 (0) |
| Patency | 66 (100) |
| Target lesion revascularization | 0 (0) |
| In-stent restenosis | 0 (0) |
- Note: Values are n (%).
- Abbreviations: CI, confidence interval; MI, myocardial infarction.
- a Per angiographic core lab.
3.4 Filter content analysis
Histomorphometric analysis was performed by an independent core laboratory on filters from 11 patients. Microscopic debris was present in 100% of filters. The Neuroguard filter collected 352% more total particles by count, and 366% more particles between 40 and 100 µm than the primary filter in these patients, as seen in Figure 3.
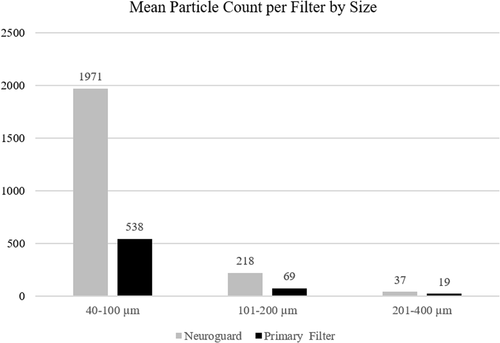
The average number of particles collected in the Neuroguard filter was significantly greater than the primary EPD for total particles per filter (2226 vs. 626, p = 0.00003). This was driven by a significantly greater capture of particles between 40 and 100 µm for the Neuroguard filter compared to the primary EPD (1971 vs. 538, p = 0.00005) and also by capture of particles >200 µm (37 vs. 18, p = 0.03). 88.5% of the total particles captured in the Neuroguard filter were ≤100 µm.
4 DISCUSSION
This study demonstrated the safety and feasibility of the Neuroguard system for the treatment of carotid artery stenosis in symptomatic and asymptomatic patients. Of the 67 patients enrolled, 11 (16.4%) of whom were symptomatic, and treated with the Neuroguard system, it is encouraging that none experienced stroke or death. Future studies should enroll a higher percentage of symptomatic patients. The primary MAE endpoint of 1.5% is significantly lower than the performance goal of 8.1%, thus indicating that this study met its performance goal. Notably, there was no occurrence of stroke in the first year, which compares favorably to contemporary CAS studies, regardless of the type of stent, embolic protection, or route of access.14, 16, 17
Procedural and technical success was achieved in 100% of patients. In aggregate, these clinical and device performance data demonstrate that the Neuroguard system with IEP is safe and can be used to successfully treat patients requiring carotid artery revascularization.
Despite improving outcomes in contemporary CAS studies14, 18 randomized trials comparing CAS to CEA have consistently shown a higher risk of minor stroke with CAS as compared to CEA.19-24 Several strategies have been proposed for reducing this risk of minor stroke, including proximal protection, alternative access, dual layer stents, and double filtration. These strategies impact the risk of 30-day stroke with CAS at various phases of the procedure, which can be broadly classified as access, stenting, and postprocedure to 30 days.
Catheter manipulation in the aortic arch to access the common carotid artery with a sheath or guide can result in embolic events, resulting in ipsilateral or contralateral strokes. Such embolic events can be avoided with appropriate patient selection and appropriate technique. Several studies have shown that with good patient selection and operator experience, the risk stroke during access is quite low, and predominantly concentrated in patient with adverse arch anatomy.15, 18 The vast majority of patients presenting with severe symptomatic or asymptomatic bifurcation carotid artery stenosis do not demonstrate adverse arch anatomy. Transradial carotid artery stenting25 and direct carotid access14 have both been used to limit catheter manipulation in the aortic arch.
Others have suggested that a significant portion of stroke risk following CAS occurs during the postprocedure phase out to 30 days.3, 26, 27 This has led to the advent of dual layered carotid stents to reduce the risk of plaque protrusion postprocedure. However, when CAS is performed with enhanced strategies for procedural embolic protection, the 30-day stroke rates are very low,2, 15, 17 suggesting that post-procedural stroke contributes minimally to the overall 30-day risk of stroke following CAS. Furthermore, these results are independent of the type of stent used, suggesting that stent type does not impact 30-day stroke risk. There are, however, reports suggesting a higher risk of stent thrombosis among dual layered stents,28 although some, but not all, of these have been reported in the setting of dual layered stents used in patients presenting with tandem lesions during acute stroke.
The predominant risk of distal embolization during CAS occurs during the stent deployment and post-dilation phases of the procedure.6, 29 Embolic protection with the integrated Neuroguard filter is enhanced as compared to traditional distal filters due to the ability to individualize the filter size and shape to suit each patient's unique anatomy, and also due to the 40 µm pore size. Double filtration, as performed in this study, likely improves the protection even further.
Histological analyses of all the particles captured in both filters showed that 72% of the total number of particles were captured in the Neuroguard filter, and the majority of these particles were less than 100 µm in size. This is consistent with data from the Paladin Registry.2 These microembolic particles may not be as efficiently captured in commercially available distal filters which have pores >100 µm in size and may contribute to the higher risk of minor stroke seen in CAS studies done with a such distal filters. The Neuroguard filter size can be customized to the individual patient's anatomy to allow circumferential apposition of the filter to the vessel wall, and with 40 µm pores within the filter, it is likely that the debris contained within the distal filter did not escape the Neuroguard filter, but rather was debris captured during the pre-dilatation phase.
Recently, transcarotid artery revascularization has been increasingly used to perform CAS, especially in the United States. The Roadster Study reported a 30-day death/stroke rate of 2.8% in an intention-to-treat analysis.14 The low event rate has been attributed to the elimination of the need to traverse the aortic arch during sheath positioning and reversal of flow during stent deployment. The lack of any strokes at one year in the Performance I Study shows that with experienced operators and proper patient selection, neurological events while traversing the aortic arch during transfemoral or transcarotid CAS can be prevented. Furthermore, double filtration with a primary filter as well as an integrated filter with 40 µm pores, which is customizable to the patient's anatomy, can minimize the risk of embolic debris from reaching the brain during stent deployment and post-dilation. Thus, with CAS performed with the Neuroguard system, patients can benefit from a fully endovascular procedure without compromising safety.
This study did not include a DW-MRI analysis, but the Paladin Study, using the same filter, assessed pre- and postprocedure DW-MRI images.2 New ipsilateral lesions were detected in 9 (30%) patients and the mean lesion volume was 0.010 cm3, results which are similar to those seen with proximal protection.30
Furthermore, by including the post-dilation balloon on the stent delivery catheter, the number of catheter exchanges is minimized. It is generally agreed upon that the longer and more complex the procedure, the higher the risk of adverse events. The Neuroguard IEP system, by simplifying the procedure by combining several steps into one step, reduces procedural duration and the corresponding procedural risk. It is notable that direct stenting was performed in 71.6% of patients, despite the very high-grade stenosis of lesions, due to the low profile of the device.
Finally, this study also demonstrates that the novel closed-cell nitinol stent used in the Neuroguard IEP System consistently maintains patency through 12 months with duplex ultrasound data indicating a patency rate of 100% (66/66) through 12 months.
This is the first study of a novel 3-in-1 carotid stent system. To date, all randomized trials comparing CAS with CEA have shown higher risk of minor stroke with CAS. The positive results of the PERFORMANCE I Trial highlight the benefit of improving procedural embolic protection with a novel integrated filter during CAS while simultaneously simplifying the procedure by minimizing device exchanges and suggest that this may be a viable strategy to significantly reduce the risk of minor stroke associated with CAS. A large pivotal study, PERORMANCE-II (NCT04201132) is currently underway to further test this hypothesis. Furthermore, this study is designed to ensure at least 20% of the enrolled population are symptomatic.
5 STUDY LIMITATIONS
This study was not randomized, all patients were treated with double filtration. There was no active control group to directly assess for a reduction in clinical or surrogate endpoints with the Neuroguard IEP System. Patients were not consecutively enrolled, and thus there may be a bias in the type and complexity of patients enrolled in the study.
6 CONCLUSIONS
In conclusion, the results from the PERFORMANCE I study show that the Neuroguard system performs as expected and has a strong safety profile with a 0% stroke and death at 30 days in the investigated patient population.
ACKNOWLEDGMENTS
The authors would like to thank Meghan Schadow, MS for medical writing assistance and David Snead, PhD for statistical support. The authors also thank all the investigators and research staff for their contributions to this project. Contego Medical, Inc (Raleigh, North Carolina).
CONFLICTS OF INTEREST
Ralf Langhoff is a consultant for Abbott Vascular, Boston Scientific, Biotronik, B. Braun, Contego Medical, Kardionet, Medtronic, and is a member of Terumo Speakers Bureau. Ivo Petrov reports grants from Amgen, Medtronic, Cardiatis, Contego Medical, Novartis, AstraZeneca, Abbott, all outside this published work. Andrej Schmidt has consulted for Abbott, Boston Scientific, and Cook. Dierk Scheinert is on an advisory board or is a consultant for Abbott, Acotec, Alvimedica, Bayer, Boston Scientific, Cook Medical, CR Bard, IVascular, Medtronic, Philips, Upstream Peripheral Technologies. Joachim Schofer is a consultant for Edwards Lifesciences. Horst Sievert has consulted with 4tech Cardio, Abbott, Ablative Solutions, Ancora Heart, Append Medical, Axon, Bavaria Medizin Technologie GmbH, Bioventrix, Boston Scientific, Carag, Cardiac Dimensions, Cardiac Success, Cardimed, Celonova, Contego, Coramaze, Croivalve, CVRx, Dinova, Edwards, Endobar, Endologix, Endomatic, Hangzhou Nuomao Medtech, Holistick Medical, Intershunt, Intervene, K2, Lifetech, Magenta, Maquet Getinge Group, Medtronic, Metavention, Mitralix, Mokita, NXT Biomedical, Occlutech, Recor, Renal Guard, Terumo, Trisol, Vascular Dynamics, Vectorious Medtech, Venus, Venock, Vivasure Medical, Vvital Biomed, Whiteswell. Elizabeth Saylors is employed by Contego Medical. Ravish Sachar has served in the last 24 months as a consultant, advisory board member for, and received compensation for, educational programs from Boston Scientific and Medtronic; has received funds for research or clinical trials from Abbott Vascular, Boston Scientific, Bard Peripheral Vascular, Microvention, W. L. Gore and Associates, Medtronic, Terumo, and Veryan; and is a major shareholder of Contego Medical. Antonio Micari serves on an advisory board for Medtronic. The remaining authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.