Late complete atrioventricular block after closure of an atrial septal defect with a gore septal occluder (GSO™)
Conflict of interest: Nothing to report.
Abstract
Temporary intermittent complete heart block (CHB) occurred the day after interventional closure of an ASD with a 30 mm Gore Septal Occluder (GSO™) in a 2 years and 11-month-old female. CHB disappeared without further treatment and stable sinus rhythm recovered within 3 days. Only short episodes of 2nd degree AV-block (Wenckebach periodicity) at rare intervals were documented in Holter-monitors the following 2 months. Eleven months after device implantation the patient suffered from long lasting episodes of CHB. Surgical removal of the device resulted in incomplete recovery of AV-conduction. Histopathological work-up of the explanted GSO showed complete endothelialization of the device and regular scar formation. One year after surgery, the child had sinus rhythm during daytime but needed VVI-pacing while sleeping. Young age, inferior localization of the defect, and use of a large device have been individual risk factors for CHB in this patient. Clinical course and histologic findings indicate that mechanical compression was the only cause for CHB. The cumulative number of reports of CHB after use of different ASD-devices supports the recommendation to postpone the intervention in asymptomatic patients to preschool-age. Early removal of a pushing device may increase the chance of complete recovery from CHB. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Atrioventricular (AV)-block is a rare but clearly recognized complication after transcatheter closure of atrial septal defect (ASD) 1-8. Compression of the AV-node as well as inflammatory foreign body reaction or scarring has been discussed as possible reasons. Deficiency of the inferior rim has been reported to be an additional risk factor 1. Transcatheter ASD closure in small children <15 kg in general is associated with an increase of previously perceived complication rates 2. Post interventional AV-conductance defects have been observed to be temporarily in some patients 1, 5, 6, but progression to complete heart block (CHB) has been reported as well 4.
CASE REPORT
The 2 years and 11-month-old female, weight 13.6 kg, was admitted electively for interventional ASD-closure to treat right heart enlargement and poor growth. With transthoracic echocardiography the length of the atrial septum was 33 mm and the size of the defect was 9–12 mm. ASD morphology was specified as a single oval secundum defect with deficient antero-superior (retroaortic) rim with extension to the inferior (Fig. 1A). Rim to the tricuspid valve was 5–7 mm (Fig. 1B). Pre-interventional ECG and Holter monitor did not show any abnormality (Fig. 2A).
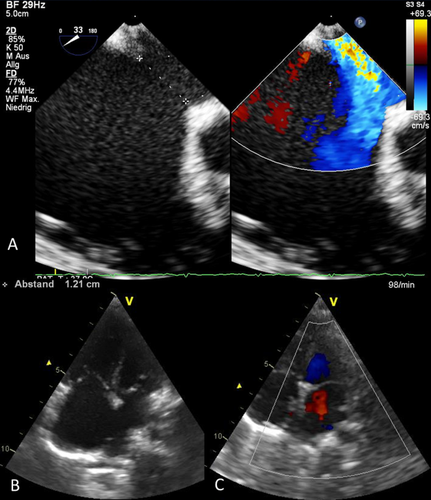
A–C: Echocardiography. A: Transesophageal echo showing the 12 mm defect at the aortic root (33 degree angulation). B: Transthoracic 4-chamber view showing the native defect. C: Relationship between device and the crux of the heart (identical pictures in several follow-up studies up to 1 year after implantation). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
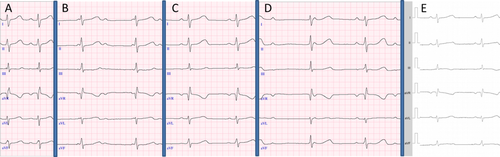
A–E: ECG. A: Pre-interventional ECG: sinus rhythm 100/min. B: 1 day after GSO implantation: 3rd degree AVB. C: 3 days after GSO implantation: sinus rhythm 75/min. D: 1 year after GSO implantation: 3rd degree AVB. E: 2 weeks after surgery: sinus rhythm 67/min. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Intervention was performed in the catheterization laboratory under conscious sedation. Native defect size was up to 12.6 mm with transesophageal echocardiography. Balloon-sizing of the defect was 13.5 mm using a 25 mm sizing catheter (pfm-medical, Köln, Germany) with the stop flow method. Due to the deficient retroaortic rim we preferred slight oversizing of the device and the ASD was closed with a 30 mm Gore Septal Occluder (GSO™, W.L. Gore & Associates, Flagstaff, AZ) without complications. Typically for the GSO device the left atrial disk after release on the delivery catheter shows a 30° angle in the a.p. (Fig. 3A) and in the lateral projection (Fig. 3B) and tilts into a 60° angle after complete release from the catheter (Fig. 3D). After release both the left and the right atrial disks show plane configuration without tension (Fig. 3C and D). At the following day continuous ECG monitoring showed intermittent periods of CHB, which were confirmed by 12 lead ECG (Fig. 2B). The ASD-device was in good position and the unloaded heart appeared much smaller (Fig. 1C) without residual shunting or compression of nearby structures. Holter monitor starting 1 day after implantation documented intermittent periods of 2nd degree heart block (2:1 conduction as well as Wenckebach-periodicity) and of 3rd degree heart block. Three days after implantation stable sinus rhythm (Fig. 2C) returned without further treatment. The patient was discharged 6 days after implantation. Only rare and short intermittent episodes of 2nd degree heart block (Wenckebach-periodicity) were documented in three Holter monitors up to 2 month after implantation. Eleven months later, the girl suffered from limited ability and was readmitted with long lasting episodes of CHB (Fig. 2D). No specific event could be requested from patient's history.
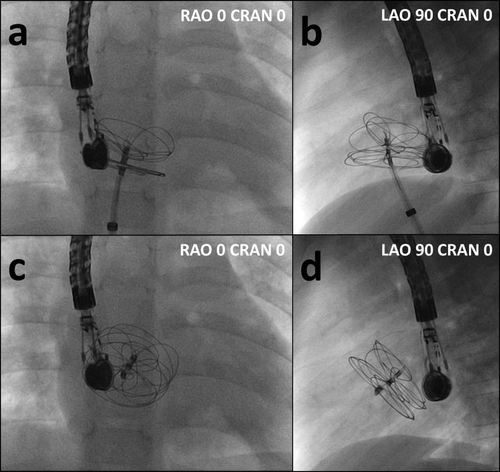
A–D: Fluoroscopy during device implantation. A: Configuration of left and right atrial disks while the device is still attached on the delivery catheter (posterior–anterior projection). B: While attached on the delivery catheter, left atrial disk props on the aortic root and forms a 30° angle (lateral projection). C: GSO after complete release, (posterior–anterior projection). D: After complete release, the disks have tilted into a 60° angle (lateral projection).
Surgery was performed via median sternotomy and in cardiac arrest. The GSO was found in correct position covering the whole ASD and remnants of the interatrial septum. The medial portion of the device was adherent to the superior part of the triangle of Koch between the coronary sinus and septal leaflet of the tricuspid valve (Fig. 4A). After smooth device extraction (Fig. 4B), the ASD was closed with an autologous pericardial patch.
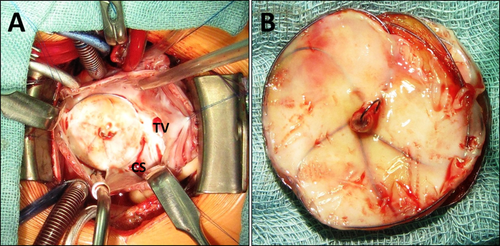
A,B: Intraoperative findings. A: Transatrial view. The medial portion of the device is adherent to the triangle of Koch. CS: coronary sinus, TV: tricuspid valve. B: Device after extraction. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The explanted GSO-specimen was analyzed in a specialized histopathologic laboratory. After fixation in formaline, the tissue block containing the device was embedded in hard resin methylmethacrylate (Technovit 9100, Kulzer & Co, Wehrheim, Germany). The resin block was subsequently sectioned in slices of 0.8 mm using a diamond band saw (300 CP, Exakt GmbH, Norderstedt, Germany). The slices were grinded down to 5–30 μm with a horizontal rotatory grinder and polisher (400 CS, Exakt GmbH, Norderstedt, Germany) and stained with Richardson Blue. Histology showed complete endothelialization of the device (Fig. 5a and b; M = metal, T = textile membrane). The membrane as well as the metal wires was surrounded by fibro-muscular tissue without infiltration of granulocytes or lymphocytes (Fig. 5c). There were only few foreign body giant cells locally related to the textile material (Fig. 5d, arrows).
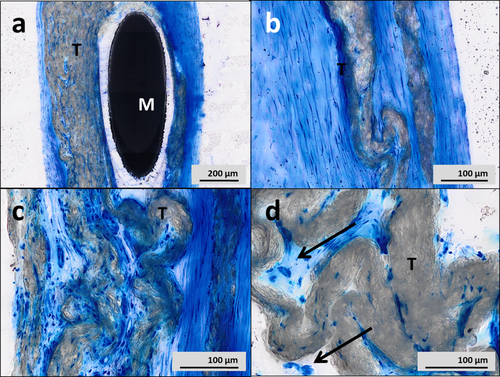
A–D: Histology of the explanted GSO. A,B: Complete endothelialization of the device (M=metal, T=textile membrane). C: No infiltration of granulocytes or lymphocytes. D: Few foreign body giant cells related to the textile membrane (arrows). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Three days after surgery, the patient still had alternating CHB and restricted circadian rhythm on Holter monitor. Two weeks later, she showed predominantly sinus rhythm only when awake (Fig. 2E). A VVI pace-maker with a permanent epicardial lead was implanted before discharge 4, 8. Six weeks later, Holter ECG documented chronotropic competence and mostly sinus rhythm at daytime, but mostly VVI pacing during the night. No further progress of AV-conductance was documented up to the latest Holter monitor 1 year after surgery.
DISCUSSION
Transcatheter device closure of atrial septal defects is a well-established method in suitable patients with good short-term and long-term results 9-11. Complication rate is lower compared to surgical closure 1 but more frequent in small children <15 kg of weight 2. CHB is a rare complication of both treatment modalities. In one large single center series 1 it occurred in 2 out of 3,000 interventional cases within 13 years. After interventional ASD closure, CHB is discussed to be caused from continued pressure or friction of atrial disks on the AV-node, leading to edema, inflammation, or scarring. Several groups have reported cases of CHB after implantation of ASD devices in the last 14 years 1-10, 12, 13. In some observations, CHB after interventional ASD closure has disappeared spontaneously 1, 5, 6 and permanently 6. The extent to which the type of device affects the risk of CHB is unknown. CHB has been described after the use of Amplatzer devices 1, 3-8 as well as after the use of Helex devices 2 and ASDOS devices 14. To our knowledge, this is the first report of CHB with the new GSO. The use of large devices in young children and a small postero-inferior rim have been discussed as risk factors for CHB 1, 3, but CHB also have occurred in patients with adequate postero-inferior margin 5.
Our case provides several aspects for the discussion of etiology and treatment of CHB.
In our case AV-block appeared early within 48 hr after implantation, like typically reported in several case descriptions and small patient series 1, 7. As in other cases 1, 5, 6 AV-conductance improved spontaneously, demonstrating structural integrity of the AV-conduction system.
AV-conduction again degenerated to CHB 4, 8 after at least 2 months of recovery as documented in Holter monitors, indicating continuing damage to the AV-node. This damage might be persistent stress, ischemia, or progressive scarring caused by foreign body reaction 1, 4.
CHB disappeared partially after removal of the device, which has covered the triangle of Koch. Together with the histopathological findings, which documented absence of inflammatory reaction, the clinical course in our patient indicates simple compression of the AV-node to be the only primary cause of CHB, which might be followed by scarring of the AV-node tissue.
In our case, the 30 mm GSO was slightly oversized to close a 12.6 mm ASD (native diameter in TEE). Though slight oversizing facilitates implantation of GSO in absent retroaortic rim and lowers the risk of embolization, the use of the smallest possible device should be considered in ASD with extension to the inferior, particularly as the unloaded heart becomes immediately smaller.
This is the first time of CHB reported after implantation of a GSO, which granted CE mark in Europe 2011 15. The GSO is built from expanded polytetrafluoroethylene film (ePTFE) covering nickel titanium (nitinol) wires. Absence of a stent and the overall design make the GSO “soft” with low radial force and low compression stress on the tissue, which is gripped between the device disks. None of the current studies on GSO have noticed AV-blocks 11, 15, 16. However, the precursor of GSO was the Helex™-device (W.L. Gore & Associates, Flagstaff, Arizona), also a “soft” device albeit it's different design. CHB has been described with a 25 mm Helex device in at least one child <15 kg 2 and another patient has been reported with non-progressive 1st degree heart block 17.
From the literature, some AV-blocks after ASD device implantation have been observed to be transiently and to respond on corticosteroid therapy in individual patients 1, 5, 6, indicating reversible edema to be part of the pathology. AV-blocks after ASD device implantation not necessarily show progression to CHB as in our case reported. However, in view of our case and the cumulative reported observations, to date we recommend considering early removal of a pushing device, if feasible the use of a smaller device or alternatively surgical closure to keep the best chances to preserve AV-conductance and to avoid the necessity of a permanent pacing system.
CONCLUSIONS
This is the first report of CHB after implantation of a GSO, using a large 30 mm device in a small child. A deficient rim to the atrio-ventricular valves and a small distance between the right atrial disk and the tricuspid valve constitute risk factors to injure the AV conductance system. Even “soft” devices like the GSO may cause enough pressure on the AV-node if they cover the Koch's triangle. Decompressing the AV-node by removing the pushing device at an early state may provide the best chance of complete recovery from CHB. In asymptomatic patients with large ASD and extension to the inferior, postponing the intervention into pre-school-age with body weight >15 kg 2 may lower the risk of CHB.
ACKNOWLEDGMENT
The authors like to thank A. Rüffer MD (Department of Pediatric Heart Surgery, Erlangen University Hospital, Germany) for providing the intraoperative pictures and description of surgical approach.