NF-κB inactivation attenuates the M1 macrophage polarization in experimental necrotizing enterocolitis by glutaredoxin-1 deficiency
Abstract
The pathogenesis of necrotizing enterocolitis (NEC) is severe inflammatory injury in preterm infants, which resulted from macrophage polarization. Nuclear factor-κB (NF-κB) is implicated to be involved in macrophage polarization. We here evaluated the essential role of NF-κB in macrophage polarization in NEC in human samples from neonates with NEC and the mouse experimental NEC model. Enhanced intestinal macrophage (IM) infiltration was presented in human neonates with NEC, the majority of which were M1 macrophages. Meanwhile, NF-κB was activated in the IMs in human NEC samples. NF-κB inhibition by BAY promoted the M1 to M2 macrophage polarization. Furthermore, glutaredoxin-1 (Grx1) deficiency promoted M2 polarization via NF-κB inactivation from the lipopolysaccharide-induced proinflammatory macrophages. The IMs isolated from Grx1−/− mice presented with decreases in total numbers and less macrophage differentiation. Grx1−/− derived IM were effective in the increased survival in experimental NEC through inflammation blocking. Our study provides evidence that NF-κB inactivation by Grx1 depletion contributed to the alleviation of NEC via inhibiting M1 macrophage polarization. The modulation to alternative macrophages in the intestines may provide a promising benefits for NEC treatment.
1 INTRODUCTION
Necrotizing enterocolitis (NEC) is the leading gastrointestinal emergency with bowel necrosis that mainly affects preterm babies (X. Li et al., 2017; Shang et al., 2017). The prominent feature of NEC include decreased splanchnic perfusion, severe inflammatory response, and bacterial colonization (Chen et al., 2019; Neu & Walker, 2011), which result in coagulative necrosis, bacterial overgrowth, and pneumatosis (Sharif et al., 2022). Histological research revealed a predominant macrophage infiltration in animal and human intestines from NEC, suggesting that a hyper inflammatory macrophage is the main feature of the innate immune system of the premature infants, which plays a critical role in the progression of NEC inflammation injury.
The infiltrating macrophages are plastic cells, which differentiated from the monocytes into two subtypes of polarized macrophages in response to microenvironmental change, including classical macrophages (M1) and alternative macrophages (M2) (Gayer et al., 2022; Shirato & Sato, 2022). Usually, the monocytes are Toll-like receptor (TLR) responsive to differentiate into inflammatory macrophages during inflammation, whereas intestinal resident macrophages are unresponsive to TLR and so presented with anti-inflammatory feature (Kim et al., 2022). We hypothesized that modulation from M1 macrophages of immune responses to M2 macrophages should be novel therapeutic strategies for NEC management (Wei & Besner, 2015).
The functions of many myeloid cells including macrophage are regulated by the transcription factor nuclear factor-κB (NF-κB), which transcripted many inflammatory genes, including chemokines, proinflammatory cytokines, cell surface receptors, and adhesion molecules. Upon NF-κB activating stimuli, such as cytokines, viruses, and lipopolysaccharide(LPS), the inhibitory κB (I-κB) kinase (IKK) β is activated to the degradation of I-κBs, resulting in NF-κB nuclear translocation, binding to NF-κB motifs, and promoting the downstream genes transcription (D. Li et al., 2022; R. Li et al., 2022; T. Zhang et al., 2021).
Previous research have suggested that NF-κB is prominently activated in the experimental NEC and NF-κB inhibition could decrease the NEC-associated intestinal injury and mortality (R. Yu et al., 2019). Furthermore, the migration and penetration of macrophages could be regulated by the NF-кB associated chemokines pathways (Yang et al., 2021).
The cytosolic oxidoreductase, glutaredoxin-1 (Grx1), is essential for modification of protein function through redox-dependent deglutathionylation of target cysteines at the expense of reduced glutathione (GSH) (X. Li et al., 2017; Shang et al., 2017). The S-glutathionylation-Grx redox modulation is proved to be critical for the magnitude of NF-кB related inflammatory signals. Grx1 increases IKKβ deglutathionylation and NF-κB activation (Jones et al., 2016; Nolin et al., 2014). The S-glutathionylation of cysteine-179 in the IKKβ is a central target for inactivation of NF-кB so play an potential role in the intestinal inflammatory disease (Aesif, Kuipers, et al., 2011; Reynaert et al., 2006).
We hypothesize that Grx1 depletion might promote the inactivation of NF-кB, which contribute to the monocyte recruitment and differentiation into proinflammatory M1 and at last confer to the protection of the NEC-like injury. We, therefore, evaluate the impact of Grx1 deficiency on macrophages polarization and whether it was in an NF-κB dependent manner in a neonatal mouse model.
2 MATERIALS AND METHODS
2.1 Samples of NEC patients
The resected necrotizing intestinal samples from NEC patients (2 mm3, n = 8) during emergency laparotomy and normal small intestines from patients with congenital intestinal atresia or imperforate anus (2 mm3, n = 6) were obtained and subjected to enzyme-linked immunosorbent assay (ELISA), western blot, and immunofluorescence analyses.
2.2 Murine experimental NEC procedures
Wild-type (WT) C57BL/6 mice were purchased from the Experimental Animal Center at Chongqing Medical University (Dr. Wenli Han). Grx1−/− mice were kindly gifted by Dr. Hongbo Luo, Boston Children's Hospital, Harvard University. Five-day-old pups from the above mice were subjected to modified NEC stress (formula gavage, hypothermia, and hypoxia), we had previously described (X. Li et al., 2017; Shang et al., 2017). Fecal samples were collected and immediately frozen daily from each animal for evaluation further. The clinical NEC manifestions were monitored, including bloody stools and abdominal distention. The final NEC diagnosis was verified by histological examination of terminal ileum. The surviving pups were euthanized 3–5 days after NEC diagnosis and the entire intestinal tract were harvested immediately upon laparotomy sacrifice. The degree of intestinal injury was measured using a standard histological scoring system as previous described. The NEC was considered if the injury score was over Grade 2. The segments were processed as appropriate for the protocols listed below, like quantitative real-time polymerase chain reaction (qRT-PCR) and western blotting.
2.3 The isolation of mouse peritoneal macrophages
The peritoneal macrophages were harvested from of the peritoneal lavage of 4–5-week-old Grx1−/− or C57BL/6 mice as described previously (MohanKumar et al., 2012). In brief, the thioglycolate broth solution (Sigma-Aldrich) was first injected intraperitoneal (ip) into the mice using 18-G needle for 3 days. The mice were then administered ip with monocyte medium to obtain the peritoneal lavage. The cells were isolated by centrifugation, spun down, and resuspended in Roswell Park Memorial Institute-1640 medium, and subsequently incubated for adherence in 12-well plastic culture plates for 2 h and the nonadherent cells were removed. The attached peritoneal macrophages were then incubated and cultured until the purity greater than 95%, then the cells were subjected to staining utilizing a Hema3 Kit (Thermo Fischer Scientific) for determinations of surface area. The surface area measurements were conducted on digital images using appropriate software package. The isolated cells were alternatively managed with LPS (100 ng/ml) in monocyte medium.
2.4 Macrophage culture and treatment
The human monocytic THP-1 cell line was purchased from ATCC and managed as previous described (MohanKumar et al., 2012). In brief, the THP-1 cells were administered with Phorbol 12-myristate 13-acetate (150 nM; Sigma-Aldrich) for macrophages differentiation. Then, the macrophages were further treated with LPS (100 ng/ml) (Sigma-Aldrich) for 6 h in their culture medium to produce M1 macrophages. Alternatively, the culture system were incubated with IKK inhibitor (BAY 11-7082; 5 µmol/L). After incubation for 24 h, all cells were isolated for immnuofluorescence staining and qRT-PCR to determined the macrophage polarization and RelA nuclear translocation.
2.5 Measurement of GSH and glutathione disulfide
The supernatant conditioned medium of peritoneal macrophages culture system were collected from each cell group and subjected to GSH and oxidized glutathione (glutathione disulfide, GSSG) analyses using the GSH/GSSG Ratio Detection Assay Kit (Abcam) as described previously (X. Li et al., 2017; Shang et al., 2017). The GSH/GSSG content was presented as nmol/mg protein. The ratio of GSH/GSSG was regarded as an index for the oxidative level.
2.6 Quantitative real-time polymerase chain reaction
The freshly dissected intestinal tissues, cultured peritoneal macrophages, and THP-1 cell line were subjected to total cell RNA extraction using TRIzol kit (Invitrogen). The complementary DNA was then reverse-transcribed from the isolated RNA using the TaqMan Reverse Transcription Reagents Kit (Applied Biosystems) and subjected to amplify with TaqMan gene-specific primers (Applied Biosystems) on an Applied Biosystems 7500 Fast Real-Time PCR System (ABI). Table 1 listed the associated the primer sequences presented in this study. The messenger RNAs (mRNAs) levels were presented after normalization to an internal control β-actin.
| Gene | Forward sequence | Reverse sequence |
|---|---|---|
| β-actin | CAGCCTTCCTTCTTGGGTAT | GCTCAGTAACAGTCCGCCTA |
| TNF-α | ATGGCCTCCCTCTCATCAGTT | ACAGGCTTGTCACTCGAATTTTG |
| IL-6 | CCAATTTCCAATGCTCTCCT | ACCACAGTGAGGAATGTCCA |
| IL-10 | CAGGCAGAGAAGCATGGC | TGCTCCACTGCCTTGCTC |
| iNOS | GTTCTCAAGGCACAGGTCTC | GCAGGTCACTTATGTCACTTATC |
| CD86 | TGGAGAGGGAAGAGAGTGAACA | GCCCATAAGTGTGCTCTGAA |
| CD206 | CCATGGACAATGCGCGAGCG | CACCTGTGGCCCAAGACACGT |
| Arg-1 | TTCTCAAAAGGACAGCCTCG | GCTCTTCATTGGCTTTCCC |
2.7 Immunofluorescence analyses
The snap-frozen slides for human intestinal tissue were first incubated in appropriate primary antibodies: an anti-CD68 antibody (the M1 macrophage marker) from Abcam and an anti-CD206 antibody (the M2 macrophage marker) from BioRad, followed by incubation with the appropriate secondary antibodies. Next, the nuclear was stained using 4′,6-diamidino-2-phenylindole (Sigma-Aldrich) and mounted with glycerol. For the murine intestinal tissues, the M1 and M2 macrophage markers were used with anti-CD68 antibody (BioRad) and anti-Arg1 antibody (Abcam), respectively. The secondary antibodies were further incubated corresponding to the primary antibodies within the dark for 50 min. During all of the staining processes, the negative control were set up to confirm the reactive specificity. The stained slides in five nonoverlapping fields per experiment were visualized with excitation at 488 nm and emission at 525 nm under fluorescence confocal microscopy (Leica Microsystems Heidelberg GmbH). The confocal images were examined for quantification in five tissue slides per pup utlizing WCIF ImageJ software (National Institutes of Health) by at least two independent investigators.
2.8 Immunofluorescence for RelA nuclear translocation
The cultured peritoneal macrophages and THP-1 cell line were managed and sedimented onto glass slides and incubated with primary antibody of RelA (Santa Cruz Biotechnology). Slides were then incubated with Alexa Fluor 568-conjugated secondary antibody. The stained cells were visualized by the above mentioned fluorescence confocal microscopy (Leica Microsystems).
2.9 Western blot
The protocol for protein extraction from snap-frozen tissues and cells were described previously (X. Li et al., 2017; Shang et al., 2017). The samples (30 μg protein for each condition) were then subjected to sodium dodecyl-sulfate polyacrylamide gel electrophoresis and membranes transfer. The following primary antibodies were used as primary antibodies: Grx1, RelA, RelB, IKKα, IκBα, PU.1, and β-actin. All antibodies were purchased from Santa Cruz Biotechnology. The primary antibodies were detected using the appropriate secondary antibodies to visualize the immunoreactive bands. The bands were reprobed with antibodies against β-actin to standardize protein loading. Relative intensities of the bands were quantified by Image J software.
2.10 Enzyme-linked immunosorbent assay
The snap-frozen human intestinal samples were homogenized for measurement the indicated cytokines by appropriate ELISA Kit (BioLegend) as described previously. The horseradish peroxidase conjugate was incubated to determine the optical densities using the microplate reader.
2.11 Primary intestinal macrophages isolation and transfer
Murine macrophages from intestine were isolated from 10-day-old mice as previously described (MohanKumar et al., 2012). In brief, intestinal segments were incubated in Hanks' balanced salt solution containing 1 mM collagenase type IV (Sigma-Aldrich) to obtain the signal cell suspension. The ACK buffer was further used to lyse the red blood cells and cell suspensions were then filtered through 70 μm cell strainers. The macrophages were selected using CD11b microbeads (Miltenyi Biotec) and adhere on plates coated with polystyrene. The isolated intestinal macrophages (IMs) were administered ip of 106 per pups 1 h before the initiation NEC procedure at the first day and the third day.
2.12 Bacterial translocation
After the pups were euthanized, the venous blood, liver, spleen, and mesenteric lymph nodes were collected from each pup in a sterile fashion, and the tissues were homogenized with sterile saline (0.1 g of each sample with 0.9 ml of sterile saline) and plated on Luria–Bertani agar in anaerobic and aerobic environments for 24 h. The colony-forming units (CFUs) were measured and considered positive when the colonies were measured at >102 CFU/g.
2.13 Endotoxin assessment
The protocol for endotoxin assessment were described previously (X. Li et al., 2017; Shang et al., 2017) using Photometric Detection Test Kits (Beijing Jinshan Science and Technology Co., Ltd.) with cutoff value of 10 pg/ml.
2.14 Intestinal permeability assay
The protocol for intestinal permeability measurement was described previously (X. Li et al., 2017; Shang et al., 2017). In brief, pups were subjected to intragastric administration of fluorescein isothiocyanate-conjugated dextran (70 kDa; Sigma-Aldrich; 40 mg per 100 g body weight) for 4 h. The pups were then killed, and blood was obtained to determine the serum fluorescence level.
2.15 Statistical analysis
The statistical calculations for the data were performed using the GraphPad Prism software (GraphPad Software Inc.). The research data are tested utilizing Mann–Whitney U tests between two groups or Mann–Whitney U tests for multiple comparison. The Kaplan–Meier survival curve was performed using the logrank (Mantel–Cox) test. The data were confirmed statistically significant with p < .05.
3 RESULTS
3.1 The polarization of IM in human NEC sample
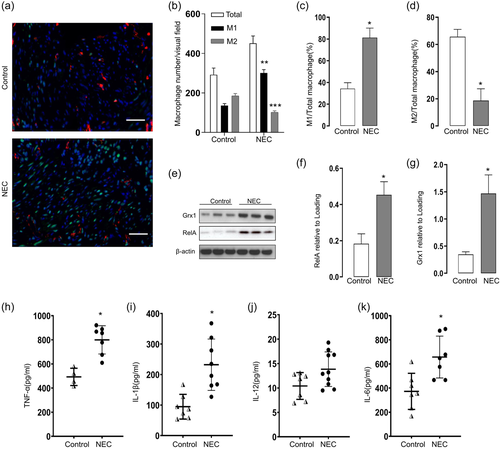
Previous research demonstrated that NEC is presented with a macrophage rich infiltration feature. We next determined macrophage polarization in the intestines of NEC sample. The immunofluorescence staining using anti-CD38 and anti-Egr2 antibodies were conducted to determine the M1 and M2 macrophages, respectively. As indicated in Figure 1a, The M1 markers positive macrophages were the majority of macrophages infiltrated in the intestines of infants developed NEC. In contrast, M2 infiltration was decreased in NEC intestine compared to the control (Figure 1b–d). We next investigated whether NF-κB activation was correlated with the macrophage M1 polarization in the infants with NEC. As expected, the presence of NF-κB (RelA) in the intestine markedly increased in the intestine sample of NEC infant (Figure 1e,f). Given that Grx1 activity could regulate macrophage polarization, we also investigated the Grx1 expression and found that increases in Grx1 content in intestine from NEC infants compared with controls via Western blot analysis (Figure 1e,g). Previous research suggested that activated macrophage are producing NF-κB dependent proinflammatory cytokines in the intestinal injury in NEC pups. We further explored the cytokine profile in the intestines from the infants with NEC. As expected, high levels of tumor necrosis factor (TNF)-α (Figure 1h), interleukin (IL)-1β (Figure 1i), IL-12 (Figure 1j), and IL-6 (Figure 1k) were observed in the intestinal from the infants with NEC.
3.2 Grx1 deficiency promotes M1 to M2 macrophage polarization in vivo
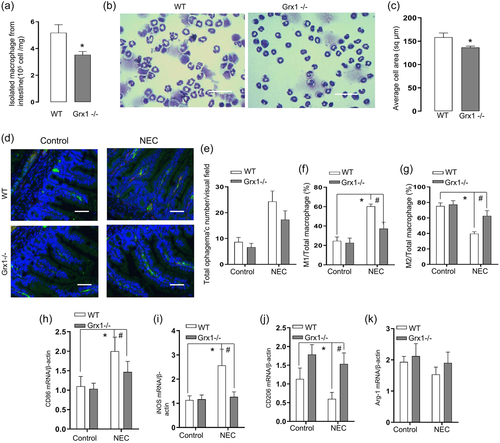
Grx1 had been implicated to be associated with the monocytic cells differentiation into macrophages (Short et al., 2016). We, therefore, next evaluated whether Grx1 deletion could influence the M1 macrophage polarization during NEC development in vivo. As indicated, the number of macrophage was reduced following the Grx1 depletion in Grx1−/− mice compared with the corresponding WT genotype (Figure 2a). Furthermore, the cell area assessment revealed that IMs isolated from Grx1−/− mice were smaller than those from WT control (Figure 2b,c). We next evaluated whether Grx1 deficiency attenuated the macrophage M1 polarization occurred in the experimental NEC intestines. As expected, total macrophage (CD68+) infiltration was increased with the the majority of M1 macrophage markers positive in the intestines of NEC pups. However, the increased M1 macrophages in the NEC was significantly abolished with Grx1 deletion (Figure 2d,e), with reduction in M1 numbers and the ratio of M1/total macrophages. In contrast, the Grx1 deficiency markedly increased M2 numbers as well as the ratio of M2/total macrophages compared to WT pups exposed to NEC (Figure 2d,f). In addition, we further assessed a panel of macrophage markers, utilizing the real time RT-PCR assessment. Consistent with our hypothesis, we found that Grx1 ablation markedly reversed the features of M1 and M2 markers, presented in the NEC pathogenesis (Figure 2g–j).
3.3 NF-κB was involved in the macrophage polarization
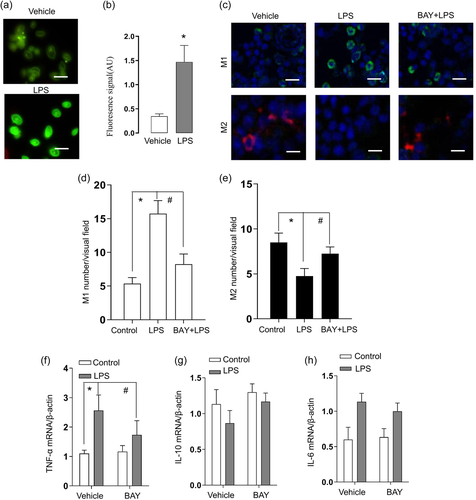
During NEC, the LPS produced by bacterial might drive macrophages polarization through NF-κB activation. We next examined the effect of LPS on NF-κB activation in cultured THP-1 cells. Consistent with this hypothesis, immunofluorescence staining analysis confirmed that LPS treatment of THP-1 macrophages induced the widespread nucleus RelA translocation from the cytoplasm (Figure 3a,b). We further investigated whether NF-κB pathway mediated the macrophage polarization. As expected, the immunofluorescence staining results confirmed that the LPS administration was accompanied with M1 macrophages increase and M2 decrease (Figure 3c,d). This effect of LPS treatment was prevented by the NF-κB inhibitor, BAY 11-7082 (Figure 3c,d). All these results suggest that the NF-κB activation induced by bacterial products promoted the neonatal gut macrophage polarization to M1 during NEC. We further evaluated the probable inflammatory cytokines following the NF-κB response in the supernatant of culture system. The results suggest that the LPS enhanced the mRNA levels of inflammatory cytokines, BAY 11-7082 suppressed the proinflammatory cytokines expression and promoted the anti-inflammatory cytokines expression (Figure 3e–g).
3.4 Grx1 deficiency is responsible for inhibiting the NF-κB pathway during macrophage polarization
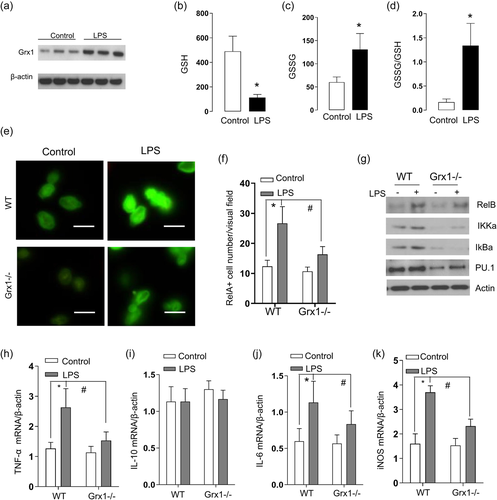
Previous research suggested that GSH adducts of IKKβ inhibit DNA binding of NF-κB and the involved chemokine production in response to LPS. We next determined contribution of Grx1 to NF-κB activity in THP-1 cells. The results in Figure 4a showed that the LPS administration induced a significant increase in Grx1 activity. We then evaluated the oxidative stress in THP-1 cells by examination the GSH and GSSG levels. As indicated in Figure 4b, LPS stress elicited a significant decrease in the GSH level and accordingly increase the GSSG content in THP-1 cells (Figure 4c). At last, the GSSG-to-GSH ratio exhibited significantly promotion in THP-1 cells following LPS administration, indicating the oxidative stress (Figure 4d). To examine the involvement of GSH adducts on the NF-κB activity further, we isolated the IMs from Grx1−/− mice, and evaluated the provocation of NF-κB under LPS stimulation. The NF-κB activation was confirmed following stimulation with LPS, in the peritoneal macrophages, as evidence of RelA nuclear translocation in the WT mice (Figure 4e). In contrast, this NF-κB activation was alleviated in peritoneal macrophages lacking Grx1 (Figure 4f, right). We further determined the NF-κB pathway components in the cytosolic extract of the involved gut macrophages by immunoblot analysis (Figure 4g). While we did not find differences in the level of RelB, IkBα, and IKKα under LPS stress, their levels were decreased in the Grx1−/− peritoneal macrophages(Figure 4g). Meanwhile, the marker of macrophage differentiation and maturity, PU.1, was evaluated using immunoblot analysis to indicate the macrophage differentiation. As indicated in Figure 4g, less expression of PU.1 was present in Grx1−/− derived peritoneal macrophages compared with peritoneal macrophages derived from WT mice, suggesting less macrophage differentiation. We also assessed the NF-κB regulated downstream cytokines levels to determine the functional consequences of Grx1 deficience. Compared with WT IMs, Grx1−/− peritoneal macrophages produced significantly less TNF-α, IL-6, IL-10, and inducible nitric oxide synthase (iNOS) in response to stimulation with LPS (Figure 4h–k).
3.5 IM lacking Grx1 relieved the severity of experimental NEC
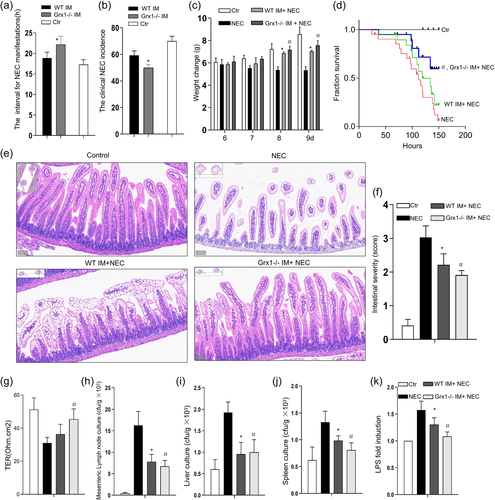
We next determined if Grx1 depletion in IM, which should be prone to M2, played a role in the manegement of experimental NEC. As indicated, the interval for the manifestations of clinical NEC were longer for the Grx1−/− IM injected mice compared to their littermate controls (Figure 5a). The clinical NEC incidences of the Grx1−/− IM injected mice were significant lower than that of control (Figure 5b). The body weight presented with a little loss for the established NEC pups compared with the control. The WT IM injection supported a weight gain of the NEC pups (Figure 5c) compared with NEC pups. Furthermore, this benefit was substantially more remarkable following the Grx1−/− IM administration (Figure 5c). In the established model of NEC, The survival curve analysis revealed a decreased survival rate for the NEC stressed mice, whereas the Grx1−/− IM injection provide a therapeutic benefit with prolonged survival rates for the NEC pups. Injection of WT IM was less effective than injection of Grx1−/− IM (Figure 5d). Next, the intestinal hematoxylin and eosin staining was evaluated for the morphologic changes. In pups following Grx1−/− IM injection, distorted microvilli integrity were attenuated and the destruction of the intestinal mucosae were substantially ameliorated, compared with that observed in the ileum of NEC mice (Figure 5e). The infiltration of inflammatory cells were strikingly fewer with the Grx1−/− IM injection then that of control. Accordingly, the pathology scores for the pups subjected to Grx1−/− IM injection were lower than that of WT IM injection (Figure 1d). Similar results were also observed when bacterial translocation was assessed (Figure 5g–k), Grx1−/− IM suppressed bacterial translocation across the intestinal barrier into the liver, the spleen and mesenteric lymph nodes, which was more efficient than that of WT IM, suggesting the functional potential of the Grx1 deficiency in promoting M2 polarization, which is advantageous to the NEC improvement.
4 DISCUSSION
Here, we demonstrated an exaggerated inflammatory response and prominent infiltration of proinflammation macrophage in the intestine in the pathogenesis of NEC. This study also presented evidence that the macrophages undergo polarization into proinflammation M1 through NF-κB activation signal pathway. NF-κB activity is suppressed by the S-glutathionylation of IKKβ at cysteine 179 site, which was modulated by Grx1. Grx1−/− peritoneal macrophages were present with immature appearance and antiimmunity function of M2, which can be leveraged to control the severe inflammation and associated intestinal injury of NEC. The above results uncovered the rationale of Grx1 for S-glutathionylation of NF-κB signaling to modulate the macrophage polarization in pathogenesis of NEC.
The premature immune system plays an essential role in the NEC pathogenesis, which causes severe intestinal inflammation (Hackam & Sodhi, 2018; Kovler et al., 2021). It is well known that the circulation monocytes contribute to the macrophage infiltration and polarization that account for the injury to the intestinal barrier function during NEC (Lee et al., 2021; Olaloye et al., 2021). We demonstrated in the current research that the macrophage-rich infiltration and their inflammatory cytokines production resulted in cellular inflammatory response in the lamina propria of intestinal tissues in neonatal mouse NEC models and human NEC (Maheshwari et al., 2011; MohanKumar et al., 2012, 2016). The macrophages have a plastic ability to switch between two functional phenotypes in response to environment changes, designated as M1 macrophages and M2 macrophages (Paoletti et al., 2022). The M1 macrophages produce proinflammatory cytokines including TNF-α, IL-1β, iNOS, and so on, so act as initiators of inflammation. In comparison, the M2 phenotype were evolutionarily originateed to promote the potent inflammation resolution, which might be a potential strategy to control the severe inflammation in various clinical conditions (Xie et al., 2022; G. Zhang et al., 2022).
The M1 macrophages has been implicated to play a essential role in pathological inflammation associated with NEC. During NEC development, the disturbed intestinal barrier function account for the bacterial translocation, which might produce higher level of LPS. The LPS is considered the classical stimuli of M1 macrophage to produce the proinflammatory cytokines (Wei & Besner, 2015; Wen et al., 2020; S. Yu et al., 2021). Previous research showed that the human intestinal epithelial cells was induced to apoptosis by M1 macrophages, which was initiated by LPS, and M2 macrophages blocked this process (Wei & Besner, 2015). In this study, we indicated that the macrophages rich feature in the intestines is presented in the NEC pups and the M1 macrophages represent the majority of infiltrated macrophages in lamina propria during NEC development, whereas the presence of M2 polarization in the NEC group was reduced compared with that observed in control. In fact, considerable studies have suggested that blocking the M1 macrophage polarization exhibited beneficial effects in various inflammatory conditions, including ulcerative colitis (Liang et al., 2022; Sun et al., 2021). Therefore, M2 polarization to promote inflammation resolution, together with the intestinal epithelial regeneration, could provide clinical benefit for these disease.
NF-κB signal have been implicated to sense danger signals, promote the recruitment and differentiation of monocytes to the intestine M1 macrophages (Qian et al., 2022). RelA, the subunit of NF-κB, translocation to the nuclear is critical for development of monocyte derived cells and maturation of myeloid cells (Bosch et al., 2019). NF-κB inactivation can promote M2 phenotype from macrophage polarization (Ai et al., 2021; Platzer et al., 2004). Indeed, the current results indicated that NF-κB activation is presented in NEC intestines and, more importantly, this NF-κB activation occurred mainly in infiltrated M1 macrophages. We further verified that the NF-κB inactivation ameliorated the overactivated inflammatory responses, and barrier dysfunction of the intestines in NEC through M2 macrophage polarization. Various inflammatory diseases such as sepsis could be relieved from the progression by NF-κB ablation in myeloid cells through inhibition of M1 macrophage polarization (Gao et al., 2022; Olivotto et al., 2021). Furthermore, a recent report suggested that NF-κB inhibition improved myocardial injury by mediating macrophage polarization (Xu et al., 2019).
The NF-κB pathway is regulated by means of IKKβ S-glutathionylation at cysteine 179. Previous research indicated that the Grx1 block account for enhancement of IKKβ S-glutathionylation and the activation of NF-κB was decreased (Jones et al., 2016; Nolin et al., 2014; Reynaert et al., 2006; Yang et al., 2021). Which NF-κB family member proteins could be potentially PSSG regulated by Grx1, although beyond the scope of the current manuscript, awaits further investigation in the future. Because the critical role of NF-κB activation in proinflammatory responses, which could be inhibited by S-glutathionylation (Aesif, Kuipers, et al., 2011), we predicted that Grx1 depletion mice should promote the alternative macrophages (M2) polarization in the pathogenesis of NEC. As expected, we indeed showed that Grx1 depletion alleviated the M1 macrophages associated inflammation response, indicating that Grx1 is critical for M1 macrophage maturation and activation. Moreover, we indicated that the NF-κB pathway protein members were suppressed by Grx1 depletion. Consistent with Grx1−/− epithelial cells, the macrophages derived from Grx1−/− mice did present the reduction of RelA nuclear translocation in response to LPS, and failed to express various inflammatory mediators.
The peritoneal macrophages derived from Grx1−/− mice presented striking features, including immature in appearance with smaller size and immature functions, like lower inflammatory cytokines productions, less PU.1 expression. Together with this, Grx1 depletion modulated the NF-κB activation during NEC, events that are perhaps linked with proinflammatory responses, which eventually contributed to protection the intestinal inflammatory injury. Indeed, Grx1 was previously reported to be associated with differentiation of monocyte-like cells to macrophage lineage (Aesif, Anathy, et al., 2011; Ahn et al., 2022). In our study, the Grx1−/− derived IMs presented potent anti-inflammatory activity than WT IMs. One of the possible explanations of this phenomenon is that the resident IMs retain their anti-inflammatory signature and the Grx1 depletion further facilitate the M2 macrophage polarization. Our research further manifested the potential therapeutic strategy of Grx1−/− derived IMs as a application of powerful anti-inflammatory activity in the management of inflammatory pathogenesis occurred in low weight infants. During the pathogenesis of NEC, many proinflammatory cytokines should be produced following the macrophage activation, which might determine the intestinal epithelial cells apoptosis, warrants examination in the future.
5 CONCLUSION
In summary, the present study suggest that NF-κB activation facilitate M1 macrophage polarization, which account for the inflammation activity and barrier dysfunction, so contribute to the NEC exacerbation. Our results also suggest that Grx1/PSSG redox module of IKKβ is essential for the functional maturation of macrophages and proinflammatory activation. Grx1 depletion promoted the PSSG, which inhibited the NF-κB pathway and contributed to M2 macrophage polarization. The results of macrophage polarization regulation might offer potential new clinical approaches for NEC management.
AUTHOR CONTRIBUTIONS
Yang Luo and Dan Mo conceived and designed the study. Hai Zhu, Hongjie Guo, and Cuilian Ye collected the data. Chunbao Guo analyzed and interpreted the data. Bailin Chen, Qin Deng, and Chun Deng wrote the manuscript or provided critical revisions that are important for the intellectual content. Lin Qiu and Chunbao Guo approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Ms Siqi Yang for academic support and Jiaren Liu at the Harvard University, USA, for help with the linguistic revision of the manuscript. This study was supported by the National Natural Science Foundation of China (No: 30973440, 30770950), and the key project of the Chongqing Natural Science Foundation (CSTC, 2008BA0021, cstc2012jjA0155).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The institutional review board (IRB) and the animal care and use committee of the Chongqing Medical University approved the study protocol for the management of human specimen (IRB, No.: CHMU2021-052). Written informed consents for the management of specimens were obtained from all the involved parents of the neonates.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




