Deltex cooperates with TRAF6 to promote apoptosis and cell migration through Eiger-independent JNK activation in Drosophila
Abstract
JNK signaling is a highly conserved signaling pathway that regulates a broad spectrum of cellular processes including cell proliferation, migration, and apoptosis. In Drosophila, JNK signaling is activated by binding of the tumor necrosis factor (TNF) Eiger to its receptor Wengen, and a conserved signaling cascade operates that culminates into activation of dual phosphatase Puckered thereby triggering apoptosis. The tumor necrosis factor receptor (TNFR) associated factor 6 (TRAF6) is an adaptor protein, which transduces the signal from TNFRs and Toll-like receptor/interleukin-1 receptor superfamily to induce a wide spectrum of cellular responses. TRAF6 also acts as the adaptor protein that mediates Eiger/JNK signaling in Drosophila. In a genetic interaction study, deltex (Dx) was identified as a novel interactor of TRAF6. Dx is well known to regulate Notch signaling in a context-dependent manner. Our data suggest that combinatorial action of Dx and TRAF6 enhances the Dx-induced wing nicking phenotype by inducing caspase-mediated cell death. Co-expression of Dx and TRAF6 also results in enhanced invasive behavior and perturbs the normal morphology of cells. The cooperative action of Dx and TRAF6 is attributed to JNK activation, which also leads to ectopic wingless (Wg) and decapentaplegic (Dpp) expression. Our results also reveal that the endocytic pathway component Rab7 may play a pivotal role in the regulation of Dx–TRAF6-mediated activation of JNK signaling. Here, we present the fact that Dx and TRAF6 together activate JNK signaling in an Eiger-independent mechanism.
Abbreviations
-
- JNK
-
- c-Jun N-terminal kinases
-
- MMP1
-
- matrix metalloproteinase-1
-
- PBS
-
- phosphate buffered saline
-
- TRAF6
-
- tumor necrosis factor receptor-associated factor 6
-
- UAS
-
- upstream activating sequence
1 INTRODUCTION
The transduction of information within the cell to produce a specific response is an essential prerequisite for metazoan development. Robust integration among a number of evolutionarily conserved signaling pathways, such as the Notch pathway, Wingless pathway,c-jun N-terminal kinase (JNK) pathway, etc., plays a pivotal role in achieving cellular homeostasis as well as pleiotropic cellular outputs. TNF–JNK signaling is an example of an essential signal transduction pathway that regulates a number of cellular processes such as cellular proliferation, migration, and apoptosis (Davis, 2000). In Drosophila, the canonical TNF–JNK signaling is activated by binding of the TNF Eiger (Egr) to its receptor Wengen (Wgn). This ligand–receptor binding initiates a conserved signaling cascade of MAP kinase superfamily kinases, namely Tak 1 (TGFβ activating kinase 1, a Jun kinase kinase kinase [JNKKK]), Hemipterous (Hep; Jun kinase kinase), and Basket (Bsk; Jun kinase). Activation of the pathway ultimately leads to the phosphorylation of the c-Jun N terminal kinase (JNK), which translocates into the nucleus and activates its downstream transcription factors Jun, Fos, and Foxo to trigger cell death (Weston & Davis, 2007). The tumor necrosis factor receptor (TNFR)-associated factor 6 (TRAF6) is an adaptor protein, which essentially helps in transduction of the signal from TNF receptors to the kinase Tak1, thereby inducing a wide spectrum of cellular responses (Chung et al., 2007; Narasimamurthy et al., 2009). Structurally, TRAF proteins have a RING finger domain at the N-terminus, several zinc-finger motifs, and a conserved TRAF domain at the C-terminus (Grech et al., 2000). Previously, we showed that TRAF6, via its C-terminus TRAF domain, binds to Notch-intracellular domain (Notch-ICD) and regulates Notch signaling outcome by interacting with another Notch pathway component Deltex (Dx) (Mishra et al., 2014).
Dx is a cytoplasmic protein that consists of two WWE domains at the N-terminal (Aravind, 2001), a conserved proline-rich motif, and C-terminal a RING-H2 finger motif (Matsuno et al., 1995). It has been demonstrated that via its N-terminal region, Dx binds to the intracellular domain of Notch and regulates its signaling activity in a context-dependent manner (Busseau et al., 1994; Dutta et al., 2017; Matsuno et al., 1995, 1998, 2002; Mishra et al., 2014; Mukherjee et al., 2005; Surabhi et al., 2015). For example, Dx alone positively regulates Notch signaling pathway by facilitating the transportation of the Notch receptor from the plasma membrane to cytoplasmic vesicles, where subsequent molecular events release Notch-ICD to translocate within the nucleus (Hori et al., 2004; Matsuno et al., 1995; Wilkin et al., 2008; Yamada et al., 2011). However, along with Kurtz, a mammalian homolog of nonvisual β-arrestin, Dx leads to ubiquitination-mediated proteasomal degradation of Notch, and thus negatively regulates the Notch pathway (Mukherjee et al., 2005). More recently, Dx has been reported to interact with an RNA-binding protein Hrp48 and the interaction resulted in downregulation of Notch signaling outcome. Additionally, it was also reported that coexpression of Dx along with Hrp48 induced severe cell death in epithelial tissues (Dutta et al., 2017).
In the present study, we demonstrated that the cooperative action of Dx and TRAF6 induced caspase-mediated cell death. We also observed that coexpression of Dx and TRAF6 resulted in an enhanced metastatic behavior and perturbation of the normal morphology of the cells. The synergistic action of TRAF6 and Dx is attributed to JNK activation to induce cell death and to enhance cell migration and invasiveness. In addition to inducing cell death and enhancing cell migration, Dx–TRAF6 activated JNK signaling also leads to ectopic Wg and Dpp expression indicating its role in cell proliferation. Our results also indicated that the endocytic pathway component Rab7 may play an important role in Dx and TRAF6-mediated activation of JNK signaling. Hereby, we present the fact that Dx and TRAF6 together profoundly influence the activation of JNK signaling in an Eiger-independent mechanism to induce both cell death and cell migration.
2 MATERIALS AND METHODS
2.1 Drosophila genetics
All fly stocks were maintained on standard cornmeal/yeast/molasses/agar medium at 25°C as per standard procedures. UAS-FLAG-Dx and dx152 were kindly provided by Spyros Artavanis Tsakonas. hsp70-flp; Act FRTy+FRTGAL4, UAS-GFP/CyO; H2YFP/TM2 flies were obtained from Enrique Martín-Blanco. UAS-Dx∆NBS, UAS-Dx∆PRM, UAS-Dx∆mRZF, UAS-Dx∆NBS-∆PRM,UAS-Dx∆NBS-mRZF were a kind gift from Kenji Matsuno. UAS-TRAF6-IR and UAS-wengen-IR were gifted by Tian Xu. Puc-lacZ/TM6B was kindly provided by Estee Kurant. UAS-eiger-IR was a generous gift from Masayuki Miura. TRE. DsRed.T4 was a kind gift from Florencio Serras.
UAS-Rab7-GFP (BL-42706), UAS-Rab7DN-GFP (BL-9778), UAS-p35, en-GAL4, and C96-GAL4, stocks were obtained from Bloomington stock centre. All crosses were performed at 25°C unless otherwise mentioned. To induce the expression of the gene in the specific domain, the GAL4-UAS binary system was used (Brand & Perrimon, 1993).
For clonal overexpression of UAS constructs, a combination of the FLP/FRT system and the GAL4/UAS system was used by using a yw hsp70-flp; Act FRT y + FRT-GAL4 UAS-GFP strain and was crossed with UAS-HA-TRAF6; UAS-FLAG-Dx line. The first instar larvae were kept at 37°C for 60 min for generating heat-shock induced clones.
Appropriate genetic crosses were designed to make the combination lines.
2.2 Immunocytochemistry and confocal microscopy
Drosophila third instar larval imaginal discs were dissected in cold phosphate-buffered saline (PBS). Fixation was done at room temperature with a 1:1 mixture of 3% paraformaldehyde in PBS and n-heptane. After 1 min, the paraformaldehyde and n-heptane mixture was replaced by 3% paraformaldehyde and 5% dimethyl sulfoxide (DMSO). The tissues were incubated at room temperature for 20 min. Washing was done four times in Tri–PBS with 1% bovine serum albumin (BSA) for 15 min each, followed by incubation for 2 h in Tri–PBS with 0.1% BSA and 8% normal goat serum. Specific primary antibodies were diluted in blocking solution and were incubated overnight at 4°C. The next day, after four washes in Tri–PBS with 0.1% BSA and 2 h blocking, tissues were incubated with a specific secondary antibody for 90 min at room temperature, followed by three washes in Tri-PBS containing 0.1% BSA and 1 wash in 1X-PBS. 4′,6-diamidino-2-phenylindole (DAPI) was added for 20 min in the dark followed by washing four times in Tri-PBS with 0.1% BSA and once in 1X-PBS for 15 min. The discs were further dissected in chilled 1X-PBS and kept in DABCO before mounting. All slides were observed under a Carl Zeiss LSM 5l0 laser scanning confocal microscope. The images were further processed with Adobe Photoshop7. The following primary antibodies were used in this study: Rat anti-DE-cadherin 1:100 (DCAD2), mouse anti-MMP1 at 1:100, mouse anti-Wg at 1:100 (all from Developmental Studies Hybridoma Bank), rabbit anti-HA at 1:25 (Santa Cruz), mouse anti-HA at 1:100 (Sigma), rabbit anti-FLAG at 1:100 (Sigma), rabbit anti-cleaved caspase3 1:100 (Cell Signalling Technology), mouse anti-β-Gal 1:100 (Promega). Rabbit anti-Dpp antibody (1:50) was kindly provided by Matthew Gibson. The secondary antibodies used in this study were anti-mouse IgG Alexa Fluor 555 conjugate, anti-Rb IgG Alexa Fluor 488 conjugate, anti-Rat IgG Alexa Fluor 488 conjugate in 1:200 dilutions.
2.3 Real-time PCR
Total RNA was isolated from the wing imaginal discs of third instar larvae by the TRIzol method and the cDNA synthesis was carried out using a commercially available kit from Applied Biosystems, as per the manufacturer's instruction. Real-time PCR reactions were set in duplicate and performed using an Applied Biosystems 7500 sequence detection system with SYBR Green PCR Master Mix (Thermo Fisher Scientific). The relative quantification of gene expression level was calculated using the  method (Schmittgen & Livak, 2008). rps17 was used as an internal control gene to normalize the fold changes in gene expression levels. Primers used in this study are as follows:
method (Schmittgen & Livak, 2008). rps17 was used as an internal control gene to normalize the fold changes in gene expression levels. Primers used in this study are as follows:
rps17:
Forward primer: 5ʹ-AAGCGCATCTGCGAGGAG-3ʹ
Reverse primer: 5ʹ-CCTCCTCCTGCAACTTGATG-3ʹ
mmp1:
Forward primer: 5ʹ-GGCAGAGGCGGGTAGATAG-3ʹ
Reverse primer: 5ʹ-TTCAGTGTTCATAGTCGTAGGC-3ʹ
wg:
Forward primer: 5ʹ-GATTATTCCGCAGTCTGGTC-3ʹ
Reverse primer: 5ʹ-CTATTATGCTTGCGTCCCTG-3ʹ
dpp:
Forward primer: 5ʹ-GCTACCAGGTGCTTGTCTACG-3ʹ
Reverse primer: 5ʹ-GTCTTGGTGTCCAACAGCAG-3ʹ
eiger:
Forward primer: 5ʹ-TAATCTCCAGCAGCGT-3ʹ
Reverse primer: 5ʹ-GTAGTCTGCGCCAACA-3ʹ
wengen:
Forward primer: 5ʹ-CTGGAAGGAGCGCCGAAAGT-3ʹ
Reverse primer: 5ʹ-GGCCACGTAAAGAACGCCAG-3ʹ.
2.4 Acridine orange staining
Third instar larval imaginal discs were dissected out and washed briefly in cold 1X-PBS. Discs were dipped in acridine orange (1 µg/ml) for 2–3 min followed by two gentle washes with 1X-PBS. Then, the discs were mounted in PBS and quickly observed under a Nikon Eclipse 80i fluorescence microscope.
2.5 Statistical analysis
Intensity profiling was done with Image J software and the graphs were produced with the help of GraphPad Prism 5 software. Each data set was repeated thrice. The error bars in the graph represent the standard deviation from the mean. One way analysis of varience (ANOVA) with Turkey's multiple comparison post-test was deployed to determine the significance of each data set. p < .05 was accepted as statistically significant.
3 RESULTS
3.1 Cooperative interaction between Dx and TRAF6 triggers apoptosis by caspase activation
Previously, we identified TRAF6 as an interacting partner of Notch and it was shown that overexpression of Dx and TRAF6 together caused depletion of Notch protein levels (Mishra et al., 2014). Additionally, we demonstrated that synergistic interaction between Dx and TRAF6 in wing imaginal discs downregulated the Notch signaling outcome, thereby inducing serration at the wing margin in the adult flies (Figure 1c1). Downregulation of these two genes together showed wing nicking in 10% (n = 200) of the flies (Figure S1) suggesting the redundancy of dx and TRAF6 that might compensate for the loss. Earlier it was shown that Dx via interacting with Heterogeneous ribonucleoprotein (Hrp48) induced wing serration by downregulating the Notch pathway, and in this study, caspase-dependent cell death was attributed to tissue loss (Dutta et al., 2017). Thus, here we questioned if the wing nicking phenotype brought about by the synergy between Dx and TRAF6 is because of cell-death. To check the extent of cell death, we co-expressed the gene(s) of our interest in the posterior region of the wing disc and carried out acridine orange (AO) staining that specifically marks the dying cells. A significant increase in the number of AO-positive cells was observed in the posterior domain of the wing imaginal discs, when both TRAF6 and Dx were expressed together, compared to that of either Dx or TRAF6 overexpressed tissue samples (Figure 1c2). Furthermore, to check whether it was a caspase-mediated cell death, we performed a gain-of-function assay, where apoptosis was inhibited by over-expressing baculovirus caspase inhibitor protein p35 in a Dx–TRAF6 co-expression background. p35 inhibits the effector caspases DrICE and Dcp-1 thereby effectively blocking the caspase-mediated cell death (Ollmann et al., 2000). The wing serration phenotype induced by TRAF6 and Dx co-expression was found to be recovered appreciably in almost 95% of the flies (n = 100); corresponding to which a decline in active caspase level was observed in the wing imaginal disc when p35 was brought together in TRAF6 and a Dx overexpression background (Figure S2B and SB1). A rescue with p35 clearly suggests the active involvement of effector caspase in TRAF6 and Dx-mediated cell death. Consistent with our initial genetic interaction studies, an appreciable enhancement in the level of active caspase was observed in the wing imaginal disc, where both TRAF6 and Dx were co-expressed (Figure 1c4). Interestingly, majority of apoptotic cells were observed in the anterior compartment of the disc, close to the A/P boundary, indicating a non-cell autonomous cell death. In contrast, cell autonomous caspase expression was observed when only Dx was overexpressed in the posterior domain of the disc (Figure 1b4) TRAF6 alone, however, failed to inflict the expression of active caspase (Figure 1a4). For a better understanding, we generated somatic clones over-expressing TRAF6 and Dx. Expression of active caspase was found to be highly elevated in the area distant from the clone (Figure 1e3). This is because apoptosis is not a passive phenomenon and propagation of apoptosis has been reported lately (Pérez-Garijo et al., 2013). The report attributes the fact to that dying cells can generate proapoptotic signals that has the potential to induce cell death in distant neighbouring cells. The non-cell autonomous caspase expression in Dx and TRAF6 overexpression background might possibly rely on apoptosis induced apoptosis (AiA). It is to be noted here that we do not rule out the possibility of the un-dead cells in the clone hub to stimulate the induction of apoptosis in the area outside to it. Our results thus indicate that TRAF6 enhances the apoptotic property of Dx and triggers caspase dependent cell death in autonomous as well as non-cell autonomous manner.
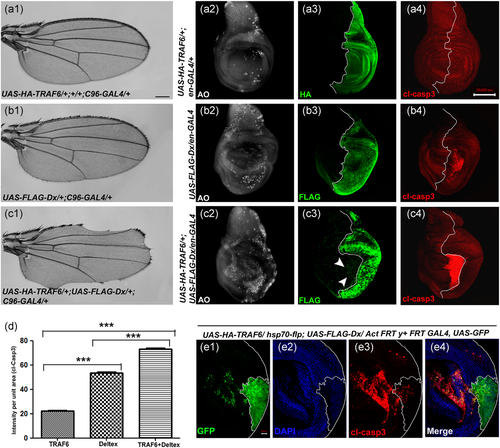
3.2 Dx together with TRAF6 induce metastatic behavior and MMP1 expression
A significant amount of cleaved caspase expression outside the posterior domain as well as in the cells distant to clone hub was surprising to us. Interestingly, it was found earlier that caspase activation also has non-apoptotic roles in both Drosophila and mammalian systems (Mukherjee & Williams, 2017; Nakajima & Kuranaga, 2017; Portela & Richardson, 2013; Ryoo et al., 2004). A non-apoptotic role for caspase activation was also revealed in cell migration in Drosophila (Rudrapatna et al., 2013). The GFP-positive cells outside the Dx and TRAF6 overexpressed clone hub (Figure 1e1) indicate the migratory nature of cells undergoing apoptosis. Matrix metalloproteinases (MMPs) have been shown to degrade the basement membrane and are required for the migratory behavior of cells in both mammals (McDonnell et al., 1991) and Drosophila (Uhlirova & Bohmann, 2006). Thus, it prompted us to check the expression level of MMP1 in the present context. Wild-type wing discs show endogenous MMP1 expression in the trachea and a small part of the notum region of the wing disc (Page-McCaw et al., 2003). Expression of Dx using the posterior compartment-specific en-GAL4 driver caused a considerable upregulation of MMP1 (Figure 2b3). In contrast, when TRAF6 was overexpressed alone, expression of MMP1 was equivalent to that of wild type, with MMP1 expression restricted to the notum and tracheal region of the wing disc (Figure 2a3). Interestingly, co-expression of TRAF6 and Dx resulted in a very high level of MMP1 expression, covering the whole of the posterior domain of the wing disc. Similar to the expression pattern of active caspase, the expression of MMP1 in wing imaginal discs, over-expressing both Dx and TRAF6 together, was not restricted only to the posterior domain of the disc. A significant amount of MMP1 expression was seen in the anterior domain of the disc too (Figure 2c3) suggesting the combinatorial effect of Dx and TRAF6 may lead to non-cell autonomous expression of MMP1. Furthermore, RT-PCR data corroborated our immuno-staining results where a significant MMP1 upregulation was also observed at the transcript level (Figure 2f).
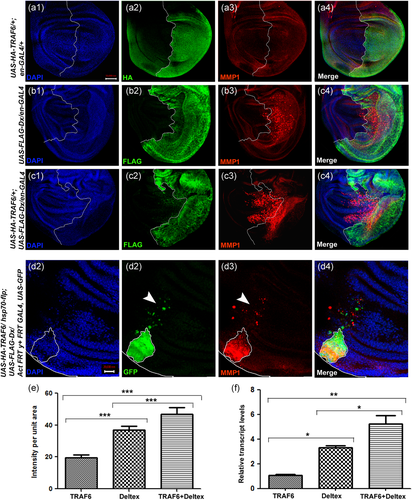
To look further in-depth, we generated somatic clones over-expressing TRAF6 and Dx together. The somatic clones showed a consistent expression pattern of MMP1 with significant MMP1 expression outside the clone hub (Figure 2d3). We also found GFP-positive cells outside the clone hub. Interestingly the GFP-positive cells and MMP1 expressing cells do not overlap, again suggesting non-cell-autonomous MMP1 expression. Our results indicate that combinatorial effect of co-expression of Dx and TRAF6 induces MMP1 expression in a cell-autonomous as well as non-cell autonomous manner.
3.3 Dx–TRAF6 expressing cells display loss of epithelial integrity
The fact that Dx and TRAF6 co-expression causes a distinct loss of epithelial integrity prompted us to check the status of specific cellular junction markers, such as cadherin and phalloidin (Leong et al., 2009; Rodahl et al., 2009; Warner et al., 2010). We overexpressed only Dx, only TRAF6, and Dx, TRAF6 together using a en-GAL4 driver and observed the expression pattern of DE-cadherin. Expression of either Dx or TRAF6 alone with the en-GAL4 driver did not cause any abnormality in the DE-cadherin expression pattern and a well-defined pattern showing a distinct cell boundary was observed (Figure 3a2,b2). However, when Dx and TRAF6 were co-expressed, a significant change in cell morphology was noticed (Figure 3c2). The cells were seemed to be elongated with a disrupted morphology. Also, the clear-cut demarcation between the anterior and posterior domain of the wing imaginal disc was lost. To strengthen the above observation, we checked the expression pattern of another cytoskeleton marker, phalloidin. It also revealed disrupted cell morphology, when both Dx and TRAF6 were expressed together, as seen in the case of cadherin expression (Figure 3c4).
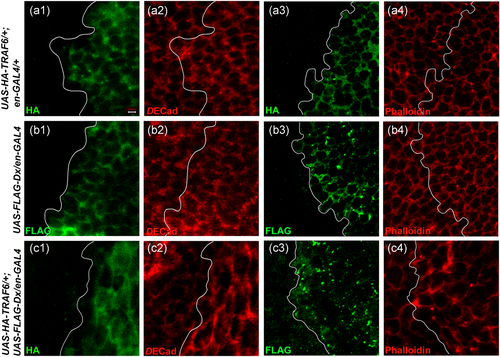
Thus, it seems that the combined action of Dx and TRAF6 is responsible for inducing cytoskeleton deformities leading to disruption of cell morphology. Such a defect in actin polymerization is typical of cells that are actively moving or undergoing shape changes (Xia & Karin, 2004). As JNK signaling is a known regulator of actin dynamics in Drosophila and other species (Xia & Karin, 2004), this hints that there might be the involvement of JNK signaling in inducing loss of epithelial integrity and metastasis.
3.4 The cooperative action of Dx and TRAF6 is linked with activated JNK signaling
In previous studies, upregulation of JNK signaling has been linked to control both apoptosis and metastatic behavior of the cell (Ho et al., 2015; Pallavi et al., 2012; Uhlirova & Bohmann, 2006). Mutation in the Drosophila tumor suppressor gene scribbled causes an upregulation of MMP1 that has been linked to JNK signaling activation (Uhlirova & Bohmann, 2006). It has also been shown that Notch co-operates with Mef2 (Myocyte enhancer factor2) to directly upregulate TNF ligand Eiger, which, in turn, leads to activation of JNK signaling (Pallavi et al., 2012). An Eiger-independent activation of JNK signaling has also been reported, where Notch co-operates with Src (Src42A and Src64B) and subsequently activates downstream MMP1 and cleaved-caspase (Ho et al., 2015).
Thus, we were also interested in determining whether apoptosis, the disrupted cell morphology, and elevated MMP1 expression mediated by Dx and TRAF synergism are related to JNK signaling. To monitor JNK activation, we used the transgenic reporter, puckered-lacZ (puc-LacZ). Puckered is a transcriptional target that acts as a feedback inhibitor of JNK (Agnès et al., 1999; Martín-Blanco et al., 1998). Expressing TRAF6 alone in the posterior domain of the wing imaginal disc using en-GAL4 showed a minimal Puckered expression (Figure 4a3). In contrast, Dx alone led to a clear upregulation of Puckered, indicating that Dx alone plays a significant role in JNK signaling upregulation (Figure 4b3), which was significantly amplified upon co-expression of Dx and TRAF6 (Figure 4c3). Similar upregulation of JNK signaling was observed when the status of TREdsRED reporter was checked in Dx and TRAF6 overexpression background (Figure S3.C3). TRE (transcriptional response element) is the regulatory sequence to which the transcription factor fos and jun binds and has been used to characterize the activation of JNK signaling (Chatterjee & Bohmann, 2012).
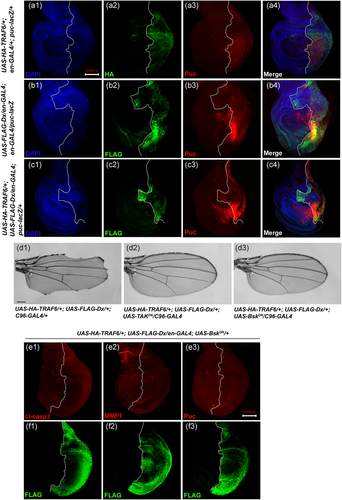
Furthermore, we questioned whether JNK signaling activation downstream to the TRAF6 and Dx synergy is a cytoplasmic kinase cascade-dependent event. The combinatorial action of Dx and TRAF6 ensued notching around the entire wing margin when driven by C96-GAL4 (Figure 4d1). We thus blocked the JNK pathway using a dominant-negative allele of the Drosophila JNK kinase gene TAK and Basket, i.e., dTAK1-DN and BasketDN (BskDN) (Adachi-Yamada et al., 1999). Strikingly, expression of either dTAK1-DN or BskDN, along with Dx and TRAF6, suppressed the notching phenotype completely (n = 100; Figure 4d2,d3). Likewise, co-expression of BskDN in Dx and TRAF6 overexpression background suppresses activated caspase expression (Figure 4E1). Expression of other JNK signaling activation markers like MMP1 and puckered were also found to be suppressed in this condition (Figure 4e2,e3). These results suggest that Dx together with TRAF6 activates a downstream cascade of events which ultimately leads to the activation of JNK signaling that, in turn, induces caspase activation and subsequent cellular outcomes.
To understand the molecular basis of Dx and TRAF6-induced JNK activation, we employed an epistatic analysis. We targeted Eiger, the sole ligand for TNF/JNK signal activation and Wengen the receptor of Eiger in Drosophila (Igaki & Miura, 2014; Igaki et al., 2002; Kauppila et al., 2003; Moreno et al., 2002). For this, we overexpressed UAS-eiger-RNAi and UAS-wengen-RNAi using C96-GAL4 in Dx and a TRAF6 co-expression background. These eiger and wengen RNAi lines were used earlier and already reported in published literature (Dutta et al., 2018; Igaki et al., 2002; Kanda et al., 2002). Moreover, we have also checked the efficiency of both the RNAi lines with Real Time PCR (Figure S4). No rescue in the wing nicking phenotype, as well as puckered expression, were observed (Figure 5b1,c1,b2), indicating that Dx and TRAF6-mediated JNK activation is independent of ligand Eiger and its receptor Wengen.
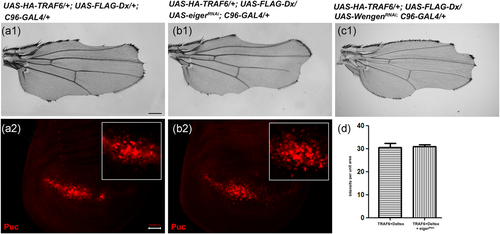
3.5 Dx–TRAF6 induced JNK activation leads to ectopic expression of wingless and decapentaplegic
Depending on the context, JNK also plays non-apoptotic roles connected with cell proliferation other than its apoptosis-inducing function (Bosch et al., 2005; Pérez-Garijo et al., 2004; Pinal et al., 2019). Earlier reports have shown that JNK signaling in apoptotic cells can induce compensatory proliferation through expression of morphogens like Wg and Dpp (Pérez-Garijo et al., 2004; Ryoo et al., 2004). It was tempting for us to check whether JNK activation due to Dx and TRAF coexpression can lead to such ectopic expression of Wg and Dpp. To investigate this further, whether Dx and TRAF6-mediated apoptosis is linked with cell proliferation, we examined the status of a morphogen, Wg together with a cell death marker, activated Caspase3. At this end, somatic clones over-expressing Dx and TRAF6 were generated and expression of activated Caspase3 and Wg was checked. The clonal area depicts elevated activated Caspase3 expression parallel to which an increase in Wg expression was observed (Figure 6a3,a4). Furthermore, we also tried to check the expression of Wg and Dpp by over-expressing Dx and TRAF6 in the posterior domain of the wing imaginal disc. We note the elevated Wg expression was mainly observed as thickening of the Wg endogenous pattern within the Dx-TRAF over-expressing domain (Figure 6b2). The elevated Dpp expression was also restricted to its endogenous domain (Figure 6c2).
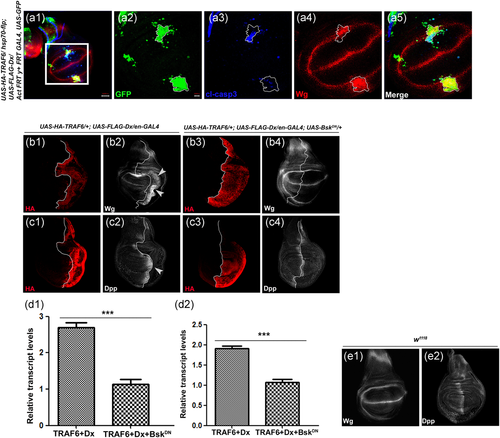
Furthermore, to check whether elevated Wg and Dpp expression is JNK dependent, we blocked the JNK signaling using a Dominant-Negative allele of Basket and checked the Wg and Dpp expression. A complete rescue in elevated Wg and Dpp expression brought about by TRAF6 and Dx cooperation was observed (Figure 6b4,c4). Here, it is conclusive that Dx and TRAF6-mediated cell proliferation through Wg and Dpp expression is indeed due to activation of JNK. RT-PCR data also corroborated our immuno-staining results where an upregulation was observed in wg and dpp transcript in a Dx and TRAF6 overexpression background and a decline in their transcript level were observed with BskDN (Figure 6d1, d2).
3.6 The notch binding domain (NBD) of Dx is critical for its interaction with TRAF6
Subsequently, to identify the domain of Dx that interacts with TRAF6, we performed an overexpression assay using different mutant lines of UAS-dx. As shown earlier, full-length Dx co-expressed with TRAF6 in the Drosophila wing under the control of C96-GAL4 induced a severe notching phenotype (Mishra et al., 2014; Figure 1a1). The overexpression of a mutant Dx lacking the nine amino acids that encompass the proline-rich motif (Dx∆PRM) or RING-H2 finger motif in combination with TRAF6 resulted in a severe notching phenotype similar to that of full-length Dx (Figure 7a,b).
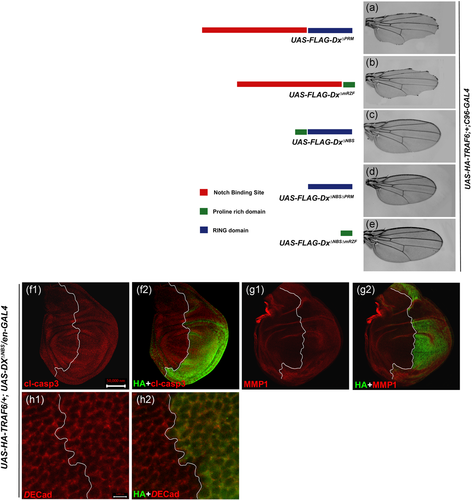
In contrast, a mutant Dx protein lacking the domain that binds to the Notch CDC10/Ankyrin repeats (Dx∆NBS) in association with TRAF6 failed to induce the notching phenotype (Figure 7c). A similar rescue in notching was obtained when both the Notch binding domain and proline-rich motif or when Dx mutant lacking both the Notch binding site and RING-H2 finger motif were brought together with TRAF6 under the control of C96-GAL4 (Figure 7d,e) and these observations suggest that Dx-NBS (Notch Binding Site) is essential for genetic interaction with TRAF6.
Furthermore, we tried to check if the absence of NBS domain from full-length Dx still has the potency to activate JNK signaling. Herein, we checked the status of different JNK associated features in the Dx mutant (lacking the NBS domain) and TRAF6 overexpression background. When the mutant form of Dx lacking the NBS domain was overexpressed along with TRAF6 in the engrailed domain of the wing imaginal disc, expression of cleaved-caspase and MMP1 was completely abolished (Figure 7f1,g1). Cell junction-associated E-cadherin also displayed normal cellular morphology (Figure 7h1). Our results prove that the NBS domain of Dx protein is indispensable for activation of the JNK signaling cascade in synergy with TRAF6.
3.7 Vesicular trafficking component Rab7 plays a pivotal role in regulating Dx-TRAF6 mediated JNK signaling
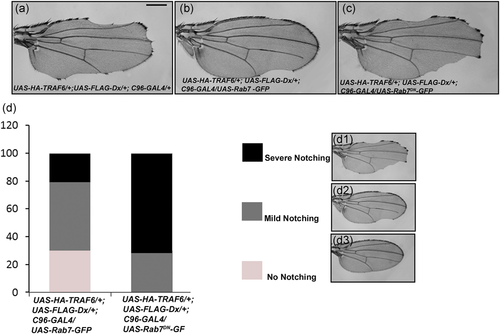
As Dx plays an important role in vesicular trafficking, we were interested in looking for the role of the transport machinery that might be involved in this Dx/TRAF6-mediated JNK activation. We performed a set of genetic interaction experiments using gain of function and dominant-negative lines of different vesicular trafficking pathway components (data not shown) and observed a significant amount of rescue in the TRAF6 and Dx-mediated wing nicking phenotype with rab7 gain of function line (Figure 8b). Depending upon the intensity of rescue in wing nicking, we divided the observed phenotype into three categories, that is, moderate notching, where the degree of nicking was relatively high; mild notching, where the degree of nicking was relatively less and no notching, where there was a complete rescue in the wing nicking phenotype (Figure 8d). It was observed that 30% of flies showed a complete rescue in the wing nicking phenotype when they were brought together with UAS-Rab7 in TRAF6 and Dx overexpression background. 50% of flies showed a mild wing nicking phenotype and 20% showed moderate notching in the wing (n = 200). When endogenous Rab7 was blocked using a dominant-negative line in the same Dx-TRAF background, there was a considerable increase in the severity in wing nicking (n = 200). In this situation, 30% of flies showed mild notching whereas 70% of flies showed moderate notching in the wing and no flies were observed without any notching in the wing (n = 200; Figure 8d). These genetic interaction data indicate that rab7 may play a significant role in TRAF6 and Dx-mediated JNK activation. However, the molecular mechanism involved in this regulation needs to be elucidated further.
4 DISCUSSION
During canonical TNF–JNK signaling activation in Drosophila, TNF Eiger (Egr) binds to its receptor Wengen (Wgn) and initiates the JNK cascade by sequentially activating TGF-β activated kinase 1 (dTAK1, a MAP kinase kinase kinase), Hemipterous (Hep, a MAP kinase kinase), and Basket (Bsk, a MAP kinase). As a result, it generates a signal that leads to cell death or cellular proliferation and migration. Dx is a cytoplasmic protein. and it has been thoroughly studied to show that Dx binds to the intracellular domain of Notch and regulates its signaling activity in a context-dependent manner (Dutta et al., 2017; Hori et al., 2004; Matsuno et al., 1995; Mishra et al., 2014; Mukherjee et al., 2005; Wilkin et al., 2008; Yamada et al., 2011). Our present study reveals that Dx synergizes with JNK-pathway component TRAF6 and induces caspase-mediated cell death. The combinatorial effect of Dx and TRAF6 was rescued when p35, an inhibitor of effector caspase was expressed in the same background. Besides this, our results show that the induction of this apoptosis is independent of the TNF ligand Eiger and the receptor Wengen. Clonal analysis further hints that the induction of apoptosis can also take place in a non-cell autonomous manner, the mechanism of which is yet to be determined.
We have previously reported that Dx has a synergistic effect on Eiger-mediated JNK activation (Dutta et al., 2018). It was shown that in coexpressed conditions, both Dx and Eiger were colocalized in the cytoplasmic vesicle. We hypothesized that Dx, being a regulator of vesicular trafficking, may facilitate the cytoplasmic relocalization of Eiger, and the endocytosed cytoplasmic Eiger non-canonically activates the JNK pathway (Dutta et al., 2018). Earlier it was also shown in a different context that Notch co-operates with myocyte enhancer factor 2 (Mef2) and synergistically activates JNK signaling through the TNF ligand Eiger (Pallavi et al., 2012). Unlike the N/Mef2 synergy, the Dx/TRAF6-mediated JNK activation presented here is independent of the ligand Eiger. A similar kind of JNK activation independent of Eiger has also been reported where Notch/Src synergy activates JNK in an Eiger-independent mechanism (Ho et al., 2015).
Increased expression of DTX and its involvement in cell migration and invasiveness in different cancers have also been reported earlier. High expression of Deltex-1 (DTX-1) was observed in follicular lymphomas, a subset of diffuse large B-cell lymphoma (DLBCL; Gupta-Rossi et al., 2003). In osteosarcoma, a variety of bone cancer, DTX-1 plays a vital role by regulating the osteosarcoma cell invasiveness via modulating Notch signaling activity (Zhang et al., 2010). In case of glioblastoma, another highly malignant primary tumor that originates in the glial cells of the central nervous system, DTX-1 acts as an oncogene by activating the noncanonical Notch pathway. In glioblastoma cells, Dx triggers other mitogenic signaling pathways such as RTK/PI3K/PKB and MAPK/ERK pathway and induces the antiapoptotic protein Mcl-1. Increased levels of DTX-1 enhance cell migration and invasiveness, thereby aggravating the severity of glioblastoma (Huber et al., 2013). Deltex-3-like (DTX3L) has been reported to be upregulated in several melanomas (Bachmann et al., 2014; Thang et al., 2015). More recently DTX3L expression was reported to be highly aggravated in melanomas than benign tumors suggesting the role of DTX3L in metastasis (Thang et al., 2015). In another set of studies, DTX3L mediates cell migration of metastatic prostate cancer (mPca) cells in a STAT1 and/or STAT3-dependent manner (Bachmann et al., 2014). Despite the several reports linking Deltex to cancer, the role of Deltex in aggravating the metastatic behavior is not yet clearly understood. TRAF mutation on the other hand is also reported in a variety of human cancers. Gain-of-function alterations are common for TRAF1, TRAF4, TRAF5, and TRAF6 in cancers. TRAF2 has evidently been seen to be associated with epithelial cancer. Indirect evidence further suggests the role of TRAF family of proteins in tumor metastasis by affecting the tumor microenvironment (Zhu et al., 2018). However, a synergistic role of Dx and TRAF6 in human cancer has not been reported yet. Ours is the first report where we postulate that Deltex interacts with TRAF6 and induces JNK signaling, in a noncanonical fashion without the initial TNF–TNFR interaction step, which, in turn, may result in the migration and invasion of malignant tumors. Thus, it would be worth exploring the status of Deltex-TRAF-induced JNK signaling activity in these malignant tumors. In addition to its apoptosis-inducing function, JNK is known to play non-apoptotic roles such as cell proliferation and cell migration. In fact, we have also seen Dx-TRAF-mediated JNK also enhances cell migration and induces ectopic expression of Wg and Dpp morphogens, indicative of its role in cell proliferation. Thus, it is intriguing to speculate that the cooperative interaction between Dx and TRAF6 may play a major role in JNK signaling activation and, in turn, oncogenesis in many human cancers.
It has been shown earlier that Src42A modulates both apoptosis and tumor invasion via Ben/dUev1a-mediated JNK activation in Drosophila (Ma et al., 2013). It was also reported that Notch/Src synergy activates JNK via a TNF-independent mechanism and leads to both cell death and enhanced metastatic behavior of cells (Ho et al., 2015). Similarly, Dx/TRAF6-mediated JNK activation results in cell death as well as enhanced cell migration and invasiveness. Our study presented here uncovers a novel Dx/TRAF6 synergy to activate JNK signaling in a TNF-independent fashion.
ACKNOWLEDGMENTS
The authors extend sincere thanks to Spyros Artavanis-Tsakonas (Harvard Medical School, USA), Kenji Matsuno (Osaka University, Japan), Estee Kurant (University of Haifa, Israel), Masayuki Miura (University of Tokyo, Japan), Florencio Serras (University of Barcelona), Matthew Gibson (University of Kansas School of Medicine), Tian Xu (Yale University School of Medicine), and the Bloomington Stock Centre for fly stocks and antibody Some of the antibodies used in the work were obtained from Developmental Studies Hybridoma Bank, University of Iowa. We also acknowledge the confocal facility of DBT-BHU-ISLS, Banaras Hindu University. Fellowship support to Vartika Sharma was provided by the Council of Scientific and Industrial Research (CSIR), Government of India.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




