H19/miR-107/HMGB1 axis sensitizes laryngeal squamous cell carcinoma to cisplatin by suppressing autophagy in vitro and in vivo
Abstract
Laryngeal squamous cell carcinoma (LSCC) is the most common malignant tumor, which occurs in the head and neck. Current treatments for LSCC are all largely weakened by increasing drug resistance. Our study aimed to investigate the effects of long noncoding RNA (lncRNA) H19 on drug resistance in LSCC. In our study, we found that the level of H19 was sharply upregulated in LSCC tissues and drug-resistant cells compared with the control. Besides, the expression of high-mobility group B1 (HMGB1) was elevated, and microRNA107 (miR-107) was suppressed in drug-resistant cells compared with the control. Further study revealed that the interference of H19 by short hairpin RNA (shRNA) effectively suppressed high autophagy level and obvious drug resistance in drug-resistant cells. Besides that, miR-107 was predicted as a target of H19 and inhibiting effects of H19 shRNA on autophagy and drug resistance were both reversed by miR-107 inhibitor. Moreover, HMGB1 was predicted as a target of miR-107 in LSCC cells and knockdown of HMGB1 was able to suppress autophagy and drug resistance in LSCC cells. In addition, our investigation demonstrated that H19 shRNA exerted an inhibiting effect on autophagy and drug resistance by downregulating HMGB1 by targeting miR-107. Finally, the in vivo experiment revealed that LV-H19 shRNA strongly suppressed drug resistance compared with the usage of cisplatin individually. Taken together, our research indicated an H19–miR-107–HMGB1 axis in regulating the autophagy-induced drug resistance in LSCC in vitro and in vivo, providing novel targets for molecular-targeted therapy and broadening the research for LSCC.
Abbreviations
-
- GFP
-
- green fluorescent protein
-
- HMGB1
-
- high mobility group box-1 protein
-
- lncRNA
-
- long noncoding RNA
-
- LSCC
-
- Laryngeal squamous cell carcinoma
-
- shRNA
-
- short hadsirpin RNA
1 INTRODUCTION
Head and neck cancer ranks sixth among the most common tumors in the world, of which laryngeal cancer has the largest proportion in head and neck cancer. Besides, about 85%–90% of larynx cancer is presented by laryngeal squamous cell carcinoma (LSCC; P. Li et al., 2017). Studies have shown that 95% of the LSCC has poor sensitivity to chemotherapy. Thus, treatment of LSCC is largely ineffective due to the resistance of the tumor cells to chemotherapy (Govindan et al., 2015). However, the resistance mechanism of LSCC cells is still unclear and the corresponding effective way to suppress drug resistance is also elusive.
Up to now, several studies have suggested that some long noncoding RNAs (lncRNAs) had a bearing on cancer development (Iyer et al., 2015). Among them, H19 RNA has attracted much attention as an important oncofetal gene. H19 was first found at chromosomal 11p15.5, and was found to play essential regulating role in the mobility of cancer cells by targeting its downstream genes (Ayesh et al., 2002; H. Li et al., 2014; Matouk et al., 2007). Besides, the effects of H19 on drug resistance have gradually become the hot spot of research. For example, K. Wu et al. (2017) reported that overexpressed H19 mediated methotrexate resistance via activating the Wnt/β-catenin signaling in colon cancer cells. Besides that, S. Chen et al. (2017) reported that H19/miR-675-5p axis regulated vitamin D receptor to induce drug resistance to 1,25(OH)2D3 in colon cancer cells. microRNAs (miRNAs) are also reported to play major effects in various cellular processes, such as proliferation, differentiation, and apoptosis (Liu et al., 2016). Among them, miRNA-107 had the ability to enhance chemosensitivity to paclitaxel by regulating Bcl-w in nonsmall cell lung cancer in vivo and in vitro (Lu et al., 2017). P. Wang et al. (2016) also reported that downregulated miR-107 promoted the progression of LSCC. However, the effects and mechanism of H19 and miR-107 on drug resistance are both ill-defined in LSCC till now.
High mobility group box-1 protein (HMGB1), also called amphoterin, plays important regulating roles in a lot of biological behaviors, such as DNA damage repair, cell replication, and cell death (Di et al., 2019). Previous studies found abnormally elevated HMGB1 in many human malignant tumors and high expression of HMGB1 was closely tied to metastasis and poor prognosis of tumors (X. Wu et al., 2018). Besides, as an important autophagy regulator, HMGB1 also participates in the therapeutic tolerance in some cancers (Chen et al., 2016). Most of the cancer cells are accompanied by HMGB1 release in the process of autophagy and apoptosis induced by external stimuli, and HMGB1 inhibition was demonstrated to sensitive cancer cells to chemotherapy or radiation. For example, miR-129-5p suppressed radiotherapy-induced autophagy in breast cancer by targeting HMGB1, thus largely improving the curative effect of radiotherapy (Luo et al., 2015). However, the effect of HMGB1 in drug resistance of LSCC remains poorly understood.
In this study, we demonstrate that an upregulated H19 was associated with autophagy-induced drug resistance in LSCC. Besides that, miR-107 was found to be a target of H19 and further investigation demonstrated that H19 regulated the expression of HMGB1 by targeting miR-107. Our study for the first time proved that the H19/miR-107/HMGB1 axis exerted regulating effects on drug resistance in LSCC in vivo and in vitro. Our work revealed a novel mechanism for drug resistance in LSCC and provided candidate therapeutic targets for LSCC treatment.
2 MATERIALS AND METHODS
2.1 Tissue specimens and cell culture
Thirty LSCC samples and 30 nontumor samples were received from the Hainan Branch of Chinese PLA General Hospital (clinical and histological characteristics of 30 LSCC patients are shown in Table 1). The study was conducted in accordance with the Helsinki Declaration, and the experiments were approved by the Ethics Committee of Hainan Branch of Chinese PLA General Hospital. All related informed consents were obtained (not public).
| Characteristics | Cases |
|---|---|
| Age (year) | |
| >60 | 17 |
| <60 | 13 |
| Sex | |
| Male | 25 |
| Female | 5 |
| Stage | |
| I–II | 9 |
| III–IV | 21 |
| Primary tumor | |
| T1–T2 | 12 |
| T3–T4 | 18 |
| Lymph node metastasis | |
| N0 | 23 |
| N1-N4 | 7 |
| Location | |
| Supraglottic | 12 |
| Glottic | 18 |
| Histology | |
| SCC I–II | 24 |
| SCC Ⅲ-Ⅴ | 6 |
Cisplatin (Selleckchem) was first dissolved by dimethyl sulfoxide and was then diluted at the concentration of 0, 5, 10, 15, 20, 40, and 60 µM for further use in cell viability detection (concentration of cisplatin was selected referencing to related research; Y. Cui et al., 2018). TU-177 cells were bought from the Cell Center of Life Science of Chinese Academy of Science (Shanghai, China). AMC-HN-8 was purchased from the American Type Culture Collection (ATCC) and was kept in our laboratory. Cisplatin-resistant TU-177 cells (TU-177/R) and AMC-HN-8/R were established by exposing TU-177 and AMC-HN-8 cells to stepwise increased concentrations of cisplatin according to previous methods (Z. Li et al., 2010). All the cells were maintained in RPMI1640 medium (Gibco) containing 10% fetal bovine serum (Gibco), penicillin, and streptomycin.
2.2 Quantitative real-time polymerase chain reaction (PCR) analysis
Total RNA in cells was extracted using TRIzol reagent (Invitrogen) and 2 μg of total RNA was synthesized into cDNA using the PrimeScript RT Reagent Kit (Takara Biotechnology). Related primers are designed and synthesized by Invitrogen and are listed as follows: H19 forward, 5′-ATCGGAGCCTCAGGGTTCGG-3′; reverse: 5′-CTGAGCTCGCCGTGACTCCG-3′; miR-107 forward, 5′-ATGAAGACCAGCATAGTACAGG-3′; reverse, 5′-GCAGCGTCCGAGCTATTC-3′. HMGB1 forward, 5ʹ-CTCGCTTCGCCAGCACA-3ʹ; reverse: 5′-AACGCTTCACGAATTAGCGT-3′. All reactions were carried out on the SYBR-Green PCR Master Mix in a Fast Real-time PCR 7500 System (Applied Biosystems). GAPDH and U6 snRNA were used as internal references of lncRNA and miRNA, respectively, and the fold change was calculated by the equation of 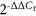 .
.
2.3 Transfection
Mimics and inhibitors (GenePharma) specific for miR-107 were transfected into TU-177 or TU-177/R cells using Lipofectamine 3000 reagent (Life Technologies Corporation) following the manufacturer's protocol.
The recombinant lentivirus for the knockdown of HMGB1 and H19 and the control lentivirus were obtained from Genechem (GenePharma). The lentiviruses were diluted into 107 transduction units (TU)/ml using 0.2 ml complete medium and were incubated together with the cells for 1 h at 37°C, followed by incubation with 0.3 ml freshly prepared RPMI1640 medium for another 24 h.
2.4 Western blot analysis
The western blot analysis was conducted according to the standard procedure. The primary antibodies (Cell Signaling Technology) used in this study are as follows: anti-Beclin-1 (1:1000), anti-LC3 I/II (1:1000), anti-Bax (1:1000), anti-Bcl-2 (1:1000), anti-HMGB1 (1:2000), and anti-GAPDH (1:2500). Then, corresponding secondary antibodies were incubated with the cells at room temperature for 1 h. An enhanced ECL system (Amersham) was used for detecting the protein signal.
2.5 Analysis of autophagy
TU-177 and TU-177/R cells were transfected with GFP-LC3 plasmid using Lipofectamine 3000™ (Invitrogen) following the manufacturer's instructions. The number of puncta formation of GFP-LC3 in cells was determined under fluorescent microscopy (The number of puncta > 5 was considered positive).
2.6 Cell viability assay
TU-177/R cells (5 × 103) seeded in 96-well plates were treated as indicated for 24 h. Cell viability was valued by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Jining Shiye) following the manufacturer's instructions. All experiments were conducted in triplicate.
2.7 Apoptosis assay by flow cytometry
Collected TU-177/R cells were washed and resuspended to a concentration of 1 × 106 cells/ml using 1 × binding buffer (BD Biosciences). Then, the cells were stained with annexin V/APC and propidium iodide using the annexin V apoptosis detection kit (KeyGen Biotech) according to the manufacturer's instructions.
2.8 Hoechst 33258 stain analysis
One hundred-microliter Hoechst 33258 reagents (Beyotime Institute of Biotechnology) were added to each well of TU-177/R cells (24-well plate, 2 × 105 cells per well) and were incubated at 37°C for 30 min in the dark. Then, the Hoechst 33258 reagents were removed and the cells washed with phosphate-buffered saline (PBS). Cells were observed under an inverted fluorescence microscope (Carl zeiss).
2.9 Bioinformatics method
Online miRNA target prediction algorithms miRanda (http://www.microrna.org/microrna/home.do) and TargetScan (http://www.targetscan.org/vert_71/) were used to analyze the potential target genes of H19 and miR-107.
2.10 Luciferase reporter assay
RNA sequence of H19 and HMGB1 mRNA 3′-UTR, which contains putative binding sites of miR-107, was inserted into pGL3 (Promega) to generate the luciferase reporter vector of pGL3-H19 or pGL3-HMGB1 wild-type (WT)/mutant-type (MUT). TU-177/R cells in 96-well plates were cotransfected with 400 ng of either 3′-UTR-WT or 3′-UTR-MUT (Promega) and 50 nmol/L miR-107 mimic Lipofectamine 3000. The Dual-Luciferase Reporter Assay System (Promega) was used to evaluate luciferase activity. The results were expressed as relative luciferase activity (firefly luciferase/Renilla luciferase).
2.11 Animal experiments
Specific pathogen-free male athymic nude mice (6–8 weeks) were obtained from the Animal Care and Use Committee of Hainan Branch of Chinese PLA General Hospital. All animal experiments were approved by the Ethics Committee of Hainan Branch of Chinese PLA General Hospital. The mice were kept in sterile conditions with free access to food and water. Cells used for tumor formation were harvested and resuspended to a final concentration of 2 × 108 cells/ml using PBS. Cells were >95% viable before injection detected by Trypan blue exclusion testing.
The mice were divided into four groups as follows and the cells were injected into either side of the flank area of each mouse by subcutaneous injection: (1) Control group: Injected with about 1 × 107 TU-177/R cells. (2) LV-H19 shRNA group: Injected with about 1 × 107 TU-177/R -LV-H19 shRNA cells. (3) Cisplatin group: Injected with about 1 × 107 TU-177/R cells. After the tumors reached 100 mm3 in size, mice of the cisplatin group were injected intraperitoneally with cisplatin (15 mg/kg) twice per week. (4) LV-H19 shRNA + cisplatin group: Injected with about 1 × 107 TU-177/R -LV-H19 shRNA cells. After the tumors reached 100 mm3 in size, mice of the cisplatin group were injected intraperitoneally with cisplatin (15 mg/kg) twice per week.
The size of the tumor was measured every 5 days and the volume of the tumor was determined according to the formula of length × width2× 0.5. Thirty days after inoculation, the mice were executed by a cervical vertebrae luxation and tumors from each mouse were randomly selected for further immunohistochemical analysis.
2.12 Immunohistochemistry (IHC)
IHC assay was performed as previously described (Guo et al., 2014). Anti-Beclin1 primary antibody (Santa Cruz) was used here.
2.13 Statistical analysis
Quantitative data were presented as mean ± SD. The differences between groups were analyzed by Student's t-test. p < .05 was considered to be statistically significant.
3 RESULTS
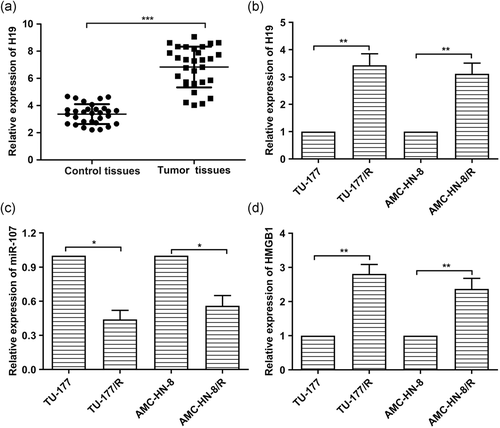
3.1 H19 and HMGB1 are highly expressed and miR-107 is lowly expressed in LSCC tissues and drug-resistant cell lines
In previous studies, some lncRNAs have been found associated with the development of cancers (Bhan et al., 2017). Similarly, we found that the expression levels of H19 were strongly increased in LSCC tissue samples compared with the adjacent normal tissues (Figure 1a). Besides, the connection between H19 expression with tumor stage, lymph node involvement, and location of the tumor was shown in the supplementary figure. Furthermore, elevated expression of H19 and HMGB1 was detected in TU-177/R cells and AMC-HN-8/R cells compared with the normal TU-177 and AMC-HN-8 cells (Figure 1b,d). At the same time, decreased expression of miR-107 was found in TU-177/R cells and AMC-HN-8/R cells compared with TU-177 and AMC-HN-8 cells (Figure 1c). Taken together, H19 and HMGB1 are highly expressed and miR-107 is lowly expressed in LSCC tissues and drug-resistant cell lines.
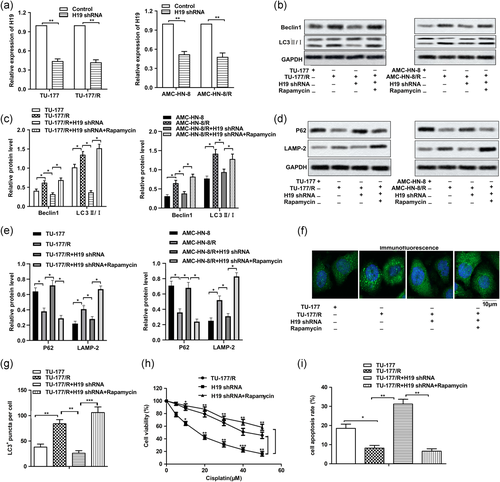
3.2 H19 shRNA suppresses autophagy and cisplatin resistance in LSCC cell lines
The underlying control of H19 on drug resistance was further investigated. Specific H19 shRNA was used to knock down the expression of H19, as shown in Figure 2a. The effect of H19 knockdown on autophagy was first examined. As shown in Figure 2b,c, more obvious autophagy was found in TU-177/R cells and AMC-HN-8/R cells compared with TU-177 and AMC-HN-8 cells. However, the induced autophagy was suppressed by the adding of H19 shRNA through down-regulating the expression of Beclin1 and inhibiting the conversion of LC3B-I to LC3B-II. The autophagy inducer Rapamycin was used as a positive control here. Then, the results in Figure 2d,e showed that the expression of P62 was elevated and the expression of LAMP-2 was suppressed in the H19 shRNA group compared with the TU-177/R group, indicating that H19 shRNA inhibited autophagy by inhibiting the fusion of autophagosomes and lysosomes. In addition, the formation of green fluorescent protein (GFP)-LC3 puncta was significantly reduced in H19 shRNA groups compared with the TU-177/R group, indicating that H19 shRNA blocked the incorporation of LC3 into autophagosomes (Figure 2f,g). Thus, further MTT assay showed that the adding of H19 shRNA obviously decreased the drug resistance compared with the TU-177/R cells group and the H19 shRNA + Rapamycin group (Figure 2h). Additionally, the results of apoptosis analysis indicated that the adding of H19 shRNA induced obvious cell apoptosis compared with the control group (Figure 2i). Taken together, suppressed H19 expression led to attenuated autophagy and drug resistance in LSCC drug-resistant cells.
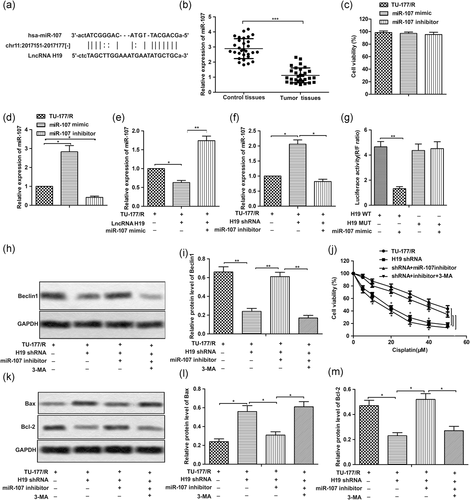
3.3 miR-107 is a target of H19 in LSCC cells
StarBase v2.0 was used to predict possible targets of H19, as shown in Figure 3a. Thus, miR-107 became our potential candidate. The expression of miR-107 was found downregulated in LSCC tissue samples compared with the adjacent normal tissues (Figure 3b). Then, specific mimic and inhibitor for miR-107 were designed and synthesized and the result of MTT assay showed there was no obvious drug toxicity in the mimic and inhibitor within our study (Figure 3c). Then, the overexpression or knockdown of miR-107 was regulated by transfecting with miR-107 mimic or miR-107 inhibitor, respectively (Figure 3d). Moreover, the level of miR-107 was decreased by lncRNA H19 and was increased by H19 shRNA (Figure 3e,f). The result of luciferase reporter assays showed that luciferase activity was much weaker in the miR-107 mimic + H19 WT group than the control group (Figure 3g). Autophagy inhibitor 3-MA was used here as a negative control. As expected, the inhibiting effect of H19 shRNA on autophagy induction in TU-177/R cells was reversed by miR-107 inhibitor by elevating the suppressed level of Beclin1 (Figure 3h,i). Similarly, the inhibiting effect of H19 shRNA on drug resistance was also counteracted by miR-107 inhibitor, as shown in Figure 3j. Moreover, proapoptotic effect of H19 shRNA was offset by miR-107 inhibitor by decreasing the level of Bax and increasing the level of Bcl-2 (Figure 3k–m). The above data together indicated that miR-107 is a target of H19 in LSCC cells and H19 shRNA inhibited drug resistance by elevating the level miR-107.
3.4 HMGB1 was a target gene of miR-107 and was also regulated by H19
Target genes for miR-107 were analyzed and HMGB1 was chosen as our research objectives (Figure 4a). Then, sharply upregulated expression of HMGB1 was found in LSCC tissue samples compared with the control (Figure 4b). The correlation analysis in Figure 4c–e showed a negative correlation between the level of miR-107 with both H19 and HMGB1 in LSCC tissue. Besides, a positive correlation was found between HMGB1with the level of H19 in LSCC tissue. The results of Figure 4f–i showed that elevated HMGB1 by lncRNA H19 was suppressed in the presence of miR-107 mimic. On the contrary, a decreased level of HMGB1 by H19 shRNA was increased in the presence of miR-107 inhibitor. In addition, we found that miR-107 overexpression largely reduced the luciferase activity in the presence of WT 3′-UTR of HMGB1 compared with the control group (Figure 4j). The above data indicated that H19 shRNA regulated the HMGB1 via elevating miR-107.
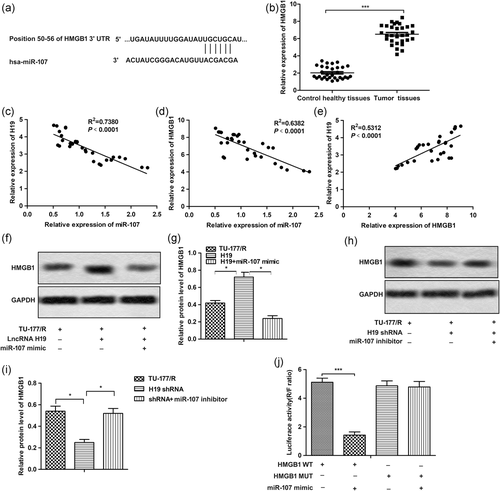
3.5 Knockdown of HMGB1 inhibited autophagy and enhanced cisplatin sensitivity
To further clarify the effect of HMGB1 on autophagy and drug resistance in LSCC cells, recombinant lentivirus carrying HMGB1 knockout gene and the control lentivirus-NC was used in our following experiments. As shown in Figure 5a,b, the expression of HMGB1 was successfully knocked down by LV-HMGB1 KD. Then, we found that the knockdown of HMGB1 suppressed high autophagy level by inhibiting the level of Beclin1 and suppressing the conversion of LC3B-I to LC3B-II in TU-177/R cells (Figures 5c,d). Besides that, the MTT assay also showed that knockdown of HMGB1 decreased miR-107 inhibitor-induced cisplatin resistance by decreasing the IC50 of TU-177/R cells from about 38 to 18 µM (Figure 5e). The results of Hoechst33342 staining showed that knockdown of HMGB1 has the same apoptosis promoting effect as H19 shRNA (Figures 5f,g). Taken together, these results indicated that knockdown of HMGB1 inhibited autophagy-associated cisplatin resistance.
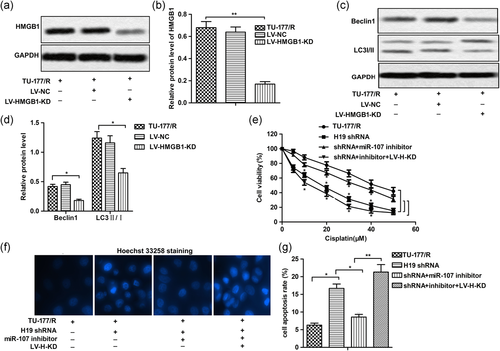
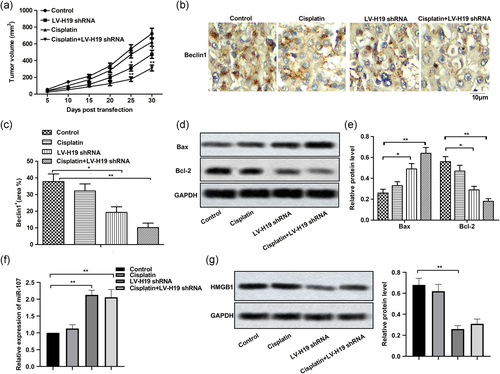
3.6 H19 knockdown inhibited autophagy-mediated drug resistance in vivo
LSCC xenograft nude mice model was constructed to explore the effect of H19 shRNA on drug resistance in the clinic. As shown in Figure 6a, cisplatin alone suppressed tumor growth compared with the control group, but there was no significant difference between them. LV-H19 shRNA had a more obvious inhibiting effect on tumor growth compared with cisplatin treatment. At the same time, the combination of cisplatin and H19 shRNA had a better anticancer effect compared with them individually. Besides that, the results of IHC showed that expression of Beclin1 was both decreased in the presence of cisplatin or H19 shRNA, especially in the cisplatin + H19 shRNA group (Figure 6b,c). Moreover, H19 shRNA also exhibited an obvious apoptosis induction effect compared with cisplatin alone (Figure 6d,e). Additionally, H19 shRNA elevated the level of miR-107 (Figure 6f) and suppressed the level of HMGB1 compared with the cisplatin group (Figure 6g,h) in vivo. Taken together, H19 knockdown inhibited autophagy-mediated drug resistance in vivo.
4 DISCUSSION
With the deepening of research, the cognition and speculation about the regulating roles of miRNAs and lncRNAs in cancer development have undergone a gradual process (Xue & He, 2014). However, there are still many unknown areas that need further exploration. Here, we aimed to investigate the effects and associated mechanism of H19 on drug resistance in LSCC.
Published studies have pointed out that lncRNAs are involved in multiple processes of tumorigenesis through a variety of mechanisms, including drug resistance (Hahne & Valeri, 2018; Sun et al., 2014). In our study, we revealed that lncRNA H19 and HMGB1 were upregulated and miR-107 was downregulated in LSCC tissues and cisplatin-resistant LSCC cells compared with the control. Similarly, previous reports reported that H19 was associated with cisplatin resistance in kinds of cancers, including ovarian cancer (Sajadpoor et al., 2018), non-small cell lung cancer (S. Chen et al., 2017) and so on. However, the effects and corresponding mechanism of H19 on drug resistance in LSCC have never been explored up to now. In our study, we revealed that the knockdown of H19 restricted drug resistance and induced obvious cell apoptosis in LSCC by suppressing autophagy.
We all know that lncRNAs exert a regulatory role by targeting corresponding downstream target genes. miRNAs are reported to exert a critical role in regulating gene expression (W. Wang et al., 2018). As reported, H19 targets miR-let-7 to facilitate the migration and invasion of tongue squamous cell carcinoma cells (Kou et al., 2019). Others also reported that H19/miR-106a-5p/E2F3 axis induced glucose metabolism and cell growth in malignant melanoma (Luan et al., 2018). In addition, an inverse association between H19 and miR-107 was demonstrated in non-small cell lung cancer by J. Cui et al. (2015). In support of this concept, we further explored the effects of the H19/miR-107 axis on drug resistance in LSCC cells. We demonstrated the negative correlation between H19 and miR-107. In addition, inhibiting effects of H19 shRNA on autophagy and drug resistance were strongly reversed by miR-107 inhibitor, identifying that H19 shRNA exhibited an inhibiting effect on autophagy and drug resistance by elevating the expression of miR-107.
Autophagy is an intracellular degradation system, which transfers the ligands of the cytoplasm to the lysosome to balance the body. Autophagy is considered to be a survival strategy under pressure, such as hypoxia and deprivation of nutrition (Mijaljica et al., 2012). Therefore, autophagy is commonly activated in tumor cells through radiotherapy and chemotherapy, thus protecting the tumor cells from the antitumor treatment and providing energy to repair the damaged DNA (Thorburn et al., 2014). Thus, the protective mechanism provided by autophagy endows tumor cells with increasing drug resistance. For example, chemotherapy drugs induced obvious autophagy and drug-resistance in breast cancer cells, and the drug resistance is markedly suppressed by autophagy inhibitors (Ambjørn et al., 2013). HMGB1 is found highly expressed in many types of tumors, such as breast cancer (Razmi et al., 2018), lung cancer (L. Wu & Yang, 2018), pancreatic cancer (Boone et al., 2018), and so on. It is found that HMGB1 enters into the cytoplasm under stress and releases Beclin-1 from the Beclin-1/Bcl-2 complex, thus inducing autophagy (Kang et al., 2010). Just as previously reported, overexpression of miR-142-3p increased chemosensitivity of NSCLC in vitro and in vivo by inhibiting HMGB1-mediated autophagy (Chen et al., 2017). However, the effects of HMGB1 on autophagy and associated drug resistance in LSCC have not been explored. In accordance with previous studies, in our study, knockdown of HMGB1 was found to suppress high autophagy and drug resistance level in LSCC cells. Further investigation found that HMGB1 was a target of miR-107 and the level of HMGB1 was regulated through H19 by targeting miR-107.
The effects of H19 on drug resistance in various xenograft models have been demonstrated. For example, Zheng et al. (2016) reported that knockdown of H19 sensitized A2780-DR cells to cisplatin in an ovarian cancer model. Besides, overexpressed H19 was reported to induce resistance of colon cancer cells to 1,25(OH)2D3 (S. Chen et al., 2017) in the xenograft model. In our study, we found that cisplatin treatment was not able to suppress tumor growth effectively in the TU-177/R cells-induced xenograft model. However, LV-H19 shRNA effectively suppressed tumor growth compared with the control group. At the same time, the combination of cisplatin and LV-H19 shRNA would get even better treatment effects. Besides that, the result of the IHC assay showed that LV-H19 shRNA suppressed the expression of autophagy marker-Beclin1 and induced apoptosis in tissues isolated from the LSCC mouse model. Also, the level of miR-107 was elevated and the level of HMGB1 was suppressed by H19 shRNA in vivo, in consonance with the in vitro experimental results.
We found that the expression level of H19 was upregulated in LSCC tissues and drug-resistant cells. Further investigation revealed that miR-107 was a target of H19. The effect of HMGB1 on autophagy-induced drug resistance has been reported by previous studies in some cancer, but the effect of HMGB1 in LSCC has not been explored. Our findings in this study indicated that H19 shRNA exerted an antidrug-resistant effect in LSCC in vitro and in vivo by regulating HMGB1 via targeting miR-107. Our studies provide new insights into the mechanism of drug resistance and novel targets for the treatment of LSCC.
ACKNOWLEDGMENTS
The authors are grateful to the Hainan Branch of Chinese PLA General Hospital, Chinese PLA General Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Liangxiang Hospital, and Weifang Medical University.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
The data and materials used in the current study are available from the corresponding author on reasonable request.




