XCT 790 is a pharmacological aggrephagy inducer in a yeast model of proteotoxicity
Abstract
Aggrephagy is a selective autophagic degradation intracellular mechanism that clears toxic misfolded protein aggregates such as α-synuclein. Here, we identify and demonstrate that the small molecule, XCT 790 alleviates α-synuclein-mediated adverse effects in a yeast model of proteotoxicity. XCT 790 induced general autophagy and also enhanced starvation-induced autophagy. Mechanistically, we showed that XCT 790 clears toxic α-synuclein aggregates in an autophagy-dependent manner. Interestingly, XCT 790 did not demonstrate a synergistic effect on autophagy induction in the presence of another autophagy inducer such as 6-Bio.
Abbreviations
-
- EGFP
-
- enhanced green fluorescent protein
-
- LOPAC
-
- library of pharmacologically active compound
-
- SNCA
-
- α-synuclein
1 INTRODUCTION
Synucleopathies are a group of neurodegenerative diseases that are characterized by the accumulation of misfolded toxic α-synuclein aggregates resulting in neuronal death (Wakabayashi et al., 2007). At the organismal level, the pace of disease manifestation positively correlates with neuronal death owing to a temporal increase in α-synuclein toxicity (Wakabayashi et al., 2007). Due to genetic factors such as duplication or triplication of SNCA gene locus (increased gene dosage), mutations such as A53T and A30P, or inefficient protein quality control machinery, α-synuclein aggregates accumulate inside neurons to exert toxic effects (Alladi et al., 2010; Suresh et al., 2017). In addition, this α-synuclein-mediated toxicity can spread through cell-to-cell transmission across neurons (Luk et al., 2012). The α-synuclein aggregates sequester intracellular proteins resulting in the compromised function of essential cellular pathways such as transcription, endocytosis, protein trafficking, and proteostatic machinery (Medeiros et al., 2018; Surguchev & Surguchov, 2017; Wong & Krainc, 2017). Clearing such toxic α-synuclein aggregates have beneficial effects at the cellular and organismal levels (Sarkar et al., 2007).
Macroautophagy (henceforth autophagy) is an intracellular degradation system that clears pathogenic micro-organisms, superfluous organelles such as peroxisomes, mitochondria, and misfolded protein aggregates (Nixon, 2013). The selective autophagy process of degrading toxic misfolded protein aggregates is called aggrephagy (Nixon, 2013). The nondividing nature of neurons makes the autophagy pathway essential for them to cope up with the accumulation of misfolded protein aggregates over time (Nixon, 2013). Thus, neuronal-specific knock out of crucial autophagy genes such as Atg5 and Atg7 resulted in the manifestation of neurodegeneration phenotypes such as the presence of ubiquitinated protein aggregates, behavioral deficits, and compromised lifespan (Hara et al., 2006; Komatsu et al., 2006). On the other hand, overexpression of the autophagy pathway enhanced the degradation of ubiquitinated protein species and extended lifespan (Pyo et al., 2013).
It is known that toxic protein aggregates hamper the activities of chaperone and ubiquitin-proteasome systems. More importantly, the aggregates also interfere with the autophagy process making them inefficient. It is reported that α-synuclein protein aggregates block autophagy at the autophagosome biogenesis stage (Winslow et al., 2010). Thus, reinstating dysfunctional autophagy by either genetic or pharmacological means help cells to cope up with the misfolded protein aggregates (Pyo et al., 2013; Rajasekhar et al., 2014, 2015; Suresh et al., 2017).
Overexpression of proteotoxic protein such as α-synuclein in Saccharomyces cerevisiae results in aggregate-mediated retardation of growth phenotype (Khurana & Lindquist, 2010). This single-celled eukaryote comprises well-conserved protein quality control machinery including autophagy that is similar to mammalian cells (Khurana & Lindquist, 2010; Suresh, Chavalmane, et al., 2018; Suresh, Verma, et al., 2018). One of the principal modes by which intracellular aggregate mediates its toxicity is by sequestering essential cellular proteins (Suresh et al., 2017; Suresh, Chavalmane, et al., 2018; Suresh, Verma, et al., 2018). This characteristic of the aggregate mode of toxicity is also recapitulated in yeast (Khurana & Lindquist, 2010).
Here, we investigated the regulation of aggrephagy pathway using the small-molecule modulator, XCT 790 in a yeast-based model of α-synuclein proteotoxicity. We tested whether XCT 790 could ameliorate the growth defect phenotype due to α-synuclein aggregates in yeast. Our results indicated that XCT 790 induces basal autophagy and promotes degradation of α-synuclein aggregates to exert cytoprotective function.
2 MATERIALS AND METHODS
2.1 Plasmid constructs
For yeast studies, the plasmids used were pSNS#5 α-synuclein-enhanced green fluorescent protein (EGFP) (in vector pRS 306) (gift from Prof. Paulo Ludovico, Portugal) under galactose promoter and pSNS#10 EGFP-Atg8 (pRS 316) (gift from Prof. Yoshinori Ohsumi, Japan).
2.2 Chemicals and antibodies used
Dextrose, peptone, yeast extract, galactose, and amino acids (uracil, methionine, histidine, lysine, and leucine) were procured from HiMedia. 6-Bio (in house synthesis), XCT 790 (X4753) was purchased from Sigma-Aldrich. The antibodies used in this study were Anti-GAPDH (MA5-15738, Thermo Scientific), Anti-Pgk1 (ab 38007, Abcam), Anti-EGFP (11 814 460 001, Roche), Anti-mouse immunoglobulin G, HRP (172-1011, Bio-Rad). CMAC-Blue (C2110) was purchased from Life Technologies.
2.3 Yeast culture conditions, α-synuclein-EGFP aggregate induction, and growth assays
Yeast media used for culturing were SD-Ura (Synthetic dextrose [2%] medium without uracil) for culturing α-synuclein-EGFP strains (WT and atg1∆) and EGFP-Atg8 processing assay, and SGal-Ura (Synthetic galactose [2%] medium without uracil) to induce α-synuclein-EGFP protein expression. The above-mentioned strains were cultured at 250 rpm and 30°C.
2.3.1 Yeast growth assays
Appropriate yeast strains were seeded (A600 ~ 0.07) with or without drugs in a 384 well plate and incubated (420 rpm, 30°C, and 80 µl) in a multiplate reader (Varioskan Flash, Thermo Scientific) for 48 h that records absorbance (A600) automatically for every 20 min. Growth curves were plotted using GraphPad Prism.
2.3.2 α-Synuclein-EGFP aggregates induction in yeast
For inducing α-synuclein-EGFP aggregates in yeast, the appropriate strains were inoculated in SD-Ura medium. Then, secondary cultures were inoculated from the primary culture and incubated until A600 reached ~0.8/ml. Cells were washed twice with sterile water and the aggregates were induced by incubating the cultures in SGal-Ura for 12–16 h.
2.4 α-Synuclein-EGFP degradation assays
After inducing α-synuclein-EGFP aggregates in the corresponding yeast strains driven by galactose promoter, the protein expression was turned-off by adding dextrose in the medium. Then, α-synuclein-EGFP aggregates degradation by XCT 790 was assessed by collecting cells treated with and without XCT 790 (50 μM) for 0 and 24 h. Subsequently, the α-synuclein-EGFP protein levels were analyzed using immunoblotting.
2.5 Immunoblot analysis
The appropriate yeast strains (A600 = 3) were mixed in trichloroacetic acid (12.5%) and then stored at −80°C. Then, the samples were thawed on ice, centrifuged (16,000 g, 15 min), and the pellets were washed twice with ice-cold acetone (80%). Pellets were air-dried, resuspended in lysis solution (0.1 N NaOH and 1% sodium dodecyl sulfate [SDS]) and Laemmli buffer, and boiled for 15 min. The lysates were electrophoresed onto SDS-polyacrylamide gel electrophoresis (8%–15%) and then transferred to polyvinylidene fluoride (PVDF) (Bio-Rad) membrane using transblot turbo (Bio-Rad). Blots were then blocked with 5% skimmed milk in phosphate-buffered saline for 1 h at room temperature. Then, the blots were probed with appropriate primary antibodies at 4°C overnight and subsequently incubated with horseradish peroxidase-conjugated secondary antibody. The chemiluminescent signals were obtained using the enhanced chemiluminescence substrate (Clarity, Bio-Rad). Images were acquired using a gel documentation system (G-Box, Syngene). The bands of interest were quantitated using ImageJ software (NIH) (Schneider et al., 2012).
2.6 Microscopy
After respective treatments, the yeast cultures were washed, seeded on an agarose pad (2%), and then imaged. Images were acquired using DeltaVision Elite widefield microscope (API, GE) with the following filters: FITC (490/20 and 529/38), TRITC (542/27 and 594/45), and Cy5 (632/22 and 676/34). Acquired images were processed using DV SoftWoRX software.
2.7 Statistical analysis
One-way or two-way analysis of variance followed by Bonferroni's post hoc test was applied to derive statistical significance. Values were expressed as mean ± SD.
3 RESULTS
3.1 XCT 790 ameliorates α-Synuclein toxicity
As observed in neurons, overexpression of α-synuclein in S. cerevisiae also causes toxic protein aggregate formation that perturbs its growth leading to cell death (Khurana & Lindquist, 2010). To identify potential small molecules that could ameliorate α-synuclein-mediated cellular toxicity, we screened a small molecule library, LOPAC1280 using the established α-synuclein toxicity yeast model (Suresh et al., 2017; Suresh, Chavalmane, et al., 2018; Suresh, Verma, et al., 2018). α-Synuclein overexpression results in severe growth defects (Khurana & Lindquist, 2010). Compounds were screened for their ability to rescue the growth lag caused by α-synuclein overexpression in yeast cells. One of the hits was the compound XCT 790 (Busch et al., 2004; Eskiocak et al., 2014), a thiadiazol acrylamide, that showed significant rescue of growth in yeast cells overexpressing α-synuclein (Figure 1a). Treating wild-type (WT) yeast cells overexpressing α-synuclein-EGFP with XCT 790 significantly rescued growth lag compared with that of untreated (~3.2-fold, WT α-syn cells; untreated vs. XCT treated, p < .001, Figure 1b). This was confirmed by analyses of other growth-related parameters such as growth rate (untreated vs. XCT 790 treated, p < .001, Figure S2a) and doubling time (untreated vs. XCT 790 treated, p < .001, Figure S2b) that showed the growth of XCT 790-treated cells were significantly improved that of untreated. Importantly, XCT 790 (50 μM) did not affect WT yeast growth (growth rate; untreated vs. XCT 790 treated, p > .05: doubling time; untreated vs. XCT 790 treated, p > .05, Figure S1 a–c).
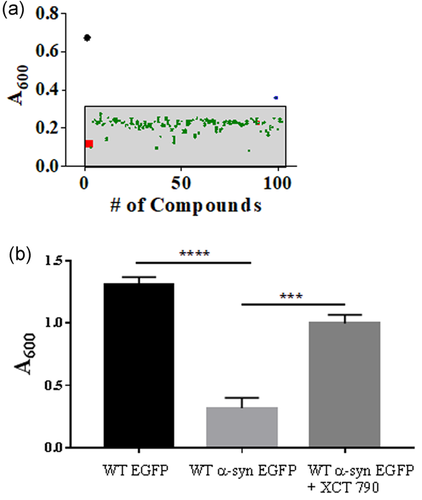
3.2 XCT 790 degrades α-Synuclein aggregates
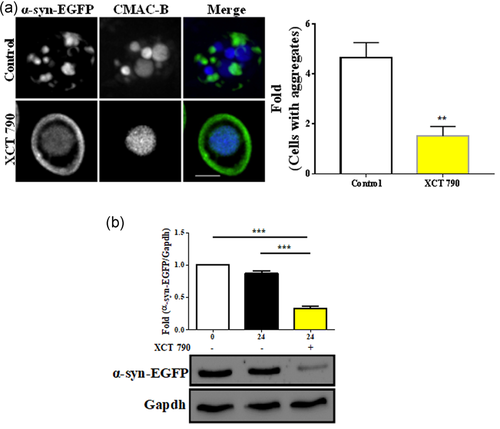
We tested whether XCT 790 treatment results in aggregate clearance. In yeast cells overexpressing α-synuclein-EGFP, XCT 790 treatment led to vacuolar degradation of α-synuclein-EGFP with the restoration of normal plasma membrane localization of α-synuclein-EGFP as seen by others (Tardiff et al., 2013) (~14-fold, untreated vs. XCT 790 treated, p < .001, Figure 2a). To validate this, we employed an α-synuclein-EGFP aggregate degradation assay in yeast. XCT 790 treatment significantly cleared α-synuclein-EGFP in the WT strain (~2.5-fold, untreated vs. XCT 790 treated, p < .001, Figures S3 and 2b). These results show that the small molecule, XCT 790 promoted vacuolar-mediated degradation of α-synuclein-EGFP aggregates.
3.3 XCT 790 induces basal autophagy and enhances starvation-induced autophagy
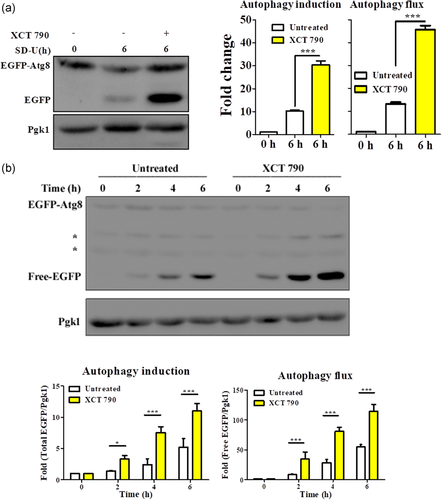
Among the many ways of coping with the toxic misfolded protein aggregates, cells employ a selective autophagy pathway known as aggrephagy to clear oligomeric misfolded proteins (Nixon, 2013). To test the involvement of autophagy-related pathways, we investigated the ability of XCT 790 to induce autophagy. We used the standard EGFP-Atg8 (an autophagosome marker) processing assay in both nutrient-rich and starvation conditions (Xie et al., 2008). This assay reveals the measure of autophagy induction (as seen by modulation of the fusion protein, EGFP-Atg8) and autophagy flux (summation of both fusion EGFP-Atg8 and free EGFP protein levels) quantitatively.
In nutrient-rich growth conditions, where basal autophagy was barely detectable, XCT 790 treatment dramatically induced autophagic flux (autophagy induction; untreated vs. XCT 790 treated, p < .001: autophagy flux; untreated vs. XCT 790 treated, p < .001, Figure 3a). Under starvation conditions, XCT 790 treatment significantly induced autophagic flux in a time-dependent manner (untreated vs. XCT 790 treated: autophagy induction: 2 h, p < .01; 4 h, p < .001; 6 h, p < .001: autophagy flux; 2 h, p < .001; 4 h, p < .001; 6 h, p < .001, Figure 3b). These results show that XCT 790 not only induced basal autophagy but also enhanced starvation-induced autophagy in yeast.
3.4 Cytoprotection and aggregate clearance potential of XCT 790 is dependent on the autophagy pathway
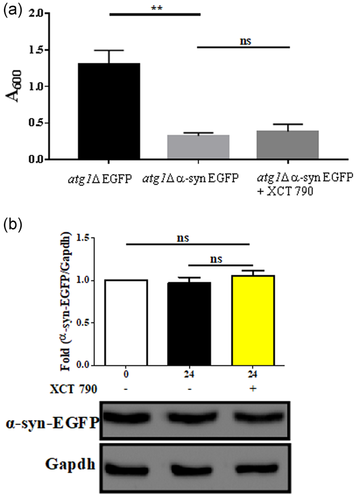
We next tested if the cytoprotection and toxic aggregate clearance abilities of XCT 790 are autophagy-dependent. Toward this, we checked for the ability of XCT 790 to rescue the growth lag in core autophagy mutant (atg1Δ) cells. Similar to WT cells, the overexpression of α-synuclein-EGFP in atg1Δ cells caused significant growth lag (atg1Δ EGFP vs. atg1Δ α-syn-EGFP cells, p < .001, Figure 4a). We observed no significant improvement in the growth of α-synuclein-EGFP overexpressing atg1Δ cells upon XCT 790 treatment (atg1Δ α-syn-EGFP cells; untreated vs. XCT 790 treated, p > .05, Figure 1b). We previously noted the significant vacuolar-mediated clearance of α-synuclein-EGFP in WT cells. In atg1Δ cells that overexpressed α-synuclein-EGFP, we did not find any significant difference in the levels of α-synuclein-EGFP upon XCT 790 treatment (untreated vs. XCT 790 treated, p > .05, Figure 4b). These results demonstrate that the XCT 790 cleared α-synuclein-EGFP to exert cytoprotective effects in an autophagy-dependent manner.
3.5 Cotreatment of XCT 790 and 6-Bio does not show a synergistic effect in autophagy induction
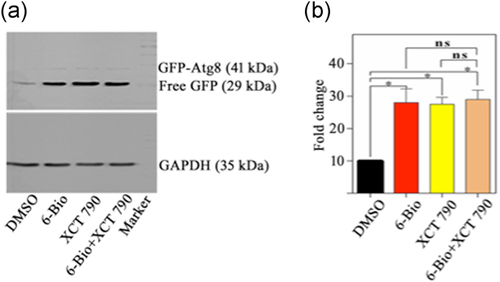
The previous studies from our lab have shown that 6-Bio ameliorates the toxic effect of α-synuclein by modulating autophagy (Suresh et al., 2017). We want to investigate the synergistic effect of 6-Bio and XCT 790 on modulating autophagy. To test the hypothesis, we treated XCT 790 (50 µM), 6-Bio (50 µM) individually as well as with the combination of XCT 790 (50 µM) and 6-Bio (50 µM) to WT yeast cells harboring EGFP-Atg8 and monitored the EGFP processing/release assay under starvation conditions. Under starvation conditions, XCT 790 only and 6-Bio only treatments significantly enhanced autophagic flux; however, the cotreatment of XCT 790 and 6-Bio did not elicit any significant synergistic effect than individual ones (untreated vs. XCT 790 treated: untreated vs. 6-Bio; untreated vs. XCT 790 + 6-Bio; 6-Bio vs. XCT 790 + 6-Bio; XCT 790 vs. XCT 790 + 6-Bio treated autophagy induction: 6 h, p < .05 [Figure 5a,b]).
4 DISCUSSION
In this study, we identified a small molecule XCT 790 that potently induces the aggrephagy process in yeast. We demonstrate that XCT 790 ameliorates α-synuclein-EGFP aggregate induced growth lag in a yeast model of proteotoxicity. This cytoprotective potential of XCT 790 occurs by degrading the toxic α-synuclein-EGFP aggregates in an autophagy-dependent manner. We used S. cerevisiae α-synuclein-EGFP proteotoxicity model that overexpressed the toxic α-synuclein aggregates (Khurana & Lindquist, 2010). This yeast model of proteotoxicity has several advantages such as short doubling time, a plethora of conserved proteostasis mechanisms, and availability of resources for their genetic manipulation (Khurana & Lindquist, 2010). From our previous small molecule screen, we identified XCT 790 as a modulator that rescued the α-synuclein-mediated growth defect (Suresh, Chavalmane, et al., 2018; Suresh, Verma, et al., 2018), and a small molecule 6-Bio modulates autophagy 6-Bio and ameliorates SNCA/a-synuclein toxicity (Suresh et al., 2017). The alleviation of α-synuclein-mediated toxicity by XCT 790 and 6-Bio occurs through degrading the toxic aggregate levels. The endogenous localization of α-synuclein in neurons is on the synaptic vesicles to mediate exocytosis and other vital functions (Burre, 2015). The small molecule not only cleared the toxic α-synuclein aggregates but also restored its normal localization on the plasma membrane in yeast (Suresh, Chavalmane, et al., 2018; Suresh, Verma, et al., 2018).
Cells are equipped with efficient protein quality control machinery such as chaperones, the ubiquitin-proteasome system, and autophagy. The toxic protein aggregates are substrates for the autophagy pathway owing to their size (Nixon, 2013). Mechanistically, we found that the small molecule XCT 790 and 6-Bio protects against α-synuclein proteotoxicity through enhancing autophagy. The autophagy dependency of XCT 790 is shown by growth and also α-synuclein-EGFP degradation assays. Our earlier studies using neuronal cell cultures and mice models indicated that XCT 790 and 6-Bio induced autophagy in those models to exert neuroprotection (Suresh et al., 2017; Suresh, Chavalmane, et al., 2018; Suresh, Verma, et al., 2018). In these studies, we also showed that there is no significant induction of autophagy by combined treatment of 6-Bio and XCT 790. Apart from clearing the toxic α-synuclein aggregates, XCT 790 itself induced general autophagy in fed conditions without affecting its growth. It not only induced basal autophagy but also augmented starvation-induced autophagy. In summary, we identified XCT 790 as a small-molecule modulator of aggrephagy in a yeast model of α-synuclein proteotoxicity, there is no significant increase induction of autophagy by the synergistic effect of 6-Bio and XCT 790.
ACKNOWLEDGMENTS
The authors thank Paulo Ludovico for the kind gift of plasmids. This study was supported by Wellcome Trust/DBT India Alliance Intermediate Fellowship (500159-Z-09-Z), DST-SERB grant (EMR/2015/001946), and JNCASR intramural funds to Ravi Manjithaya. S. N. Suresh and Monala Jayaprakash Rao are supported by Research Associate intramural funds JNCASR. Application of XCT 790 in autophagy modulation and neurodegeneration is patent pending.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Ravi Manjithaya and S. N. Suresh conceived the project and designed the experiments. S. N. Suresh and Monala Jayaprakash Rao carried out the experiments. Ravi Manjithaya and S. N. Suresh analyzed the results and wrote the manuscript.




