Carbon tetrachloride suppresses ER-Golgi transport by inhibiting COPII vesicle formation on the ER membrane in the RLC-16 hepatocyte cell line
Abstract
Carbon tetrachloride (CCl4) causes hepatotoxicity in mammals, with its hepatocytic metabolism producing radicals that attack the intracellular membrane system and destabilize intracellular vesicle transport. Inhibition of intracellular transport causes lipid droplet retention and abnormal protein distribution. The intracellular transport of synthesized lipids and proteins from the endoplasmic reticulum (ER) to the Golgi apparatus is performed by coat complex II (COPII) vesicle transport, but how CCl4 inhibits COPII vesicle transport has not been elucidated. COPII vesicle formation on the ER membrane is initiated by the recruitment of Sar1 protein from the cytoplasm to the ER membrane, followed by that of the COPII coat constituent proteins (Sec23, Sec24, Sec13, and Sec31). In this study, we evaluated the effect of CCl4 on COPII vesicle formation using the RLC-16 rat hepatocyte cell line. Our results showed that CCl4 suppressed ER-Golgi transport in RLC-16 cells. Using a reconstituted system of rat liver tissue-derived cytoplasm and RLC-16 cell-derived ER membranes, CCl4 treatment inhibited the recruitment of Sar1 and Sec13 from the cytosolic fraction to ER membranes. CCl4-induced changes in the ER membrane accordingly inhibited the accumulation of COPII vesicle-coated constituent proteins on the ER membrane, as well as the formation of COPII vesicles, which suppressed lipid and protein transport between the ER and Golgi apparatus. Our data suggest that CCl4 inhibits ER-Golgi intracellular transport by inhibiting COPII vesicle formation on the ER membrane in hepatocytes.
Abbreviations
-
- BFA
-
- brefeldin A
-
- CCl4
-
- carbon tetrachloride
-
- COPII
-
- coat complex II
-
- ER
-
- endoplasmic reticulum
-
- PCTVs
-
- prechylomicron transport vesicles
-
- Sar1
-
- secretion-associated RAS-related protein 1
1 INTRODUCTION
The chemical compound carbon tetrachloride (CCl4) was widely used worldwide until the 1970s as an industrial solvent, fire extinguisher, and coolant, among other uses. CCl4 has low decomposability and its residue in the environment is problematic (Dassarma et al., 2017). In mammalians, CCl4 induces hepatotoxicity secondary to lipid droplet accumulation in the hepatocyte cytoplasm. Radicals generated by hepatocytic metabolism of CCl4 modulate intracellular fat metabolism and cause lipid accumulation in the cytosol (Moody et al., 1990). CCl4-derived radicals induced by lipid peroxidation of intracellular membranes are thought to destabilize intracellular transport and lead to protein dislocation (Fujisawa et al., 2013; Newberry et al., 2019). However, the details of the molecular mechanisms involved remain unclear.
In the hepatocyte, lipids such as triglycerides synthesized in the endoplasmic reticulum (ER), as well as proteins, are transported from the ER to the Golgi apparatus (Jones et al., 2003). This process relies on coat complex II (COPII) transport vesicles (Kurokawa & Nakano, 2019; Levic et al., 2015).
COPII transport vesicles are coated by COPII coat proteins such as Sec23, Sec24, Sec13, and Sec31. COPII coat proteins drive the formation of transport vesicles at the ER membrane (Aridor et al., 1998; Bannykh et al., 1996). The assembly of COPII vesicles is initiated by recruitment of the small GTPase Sar1 from the cytosol to the ER membrane (Barlowe & Schekman, 1993; Kuge et al., 1994). In the next step, the Sec23/Sec24 complex is recruited from the cytosol to the ER membrane to form the inner COPII coat (Yoshihisa et al., 1993). Finally, the Sec13/Sec31 complex is recruited from the cytosol to the ER membrane, forming the outer COPII coat (Lederkremer et al., 2001).
In the present study, we hypothesized that CCl4-induced ER membrane damage regulates COPII vesicle assembly machinery and COPII vesicle transport and results in the accumulation of lipids in the ER lumen. While testing this hypothesis, we determined that CCl4 inhibits COPII vesicle transport in the RLC-16 rat hepatocyte cell line, which would suggest that synthesized lipids accumulate in the ER lumen rather than being exported by COPII vesicles. We found that CCl4 inhibited Sar1 and COPII coat protein recruitment during COPII vesicle formation on the ER membrane and that CCl4 suppressed COPII vesicle transport in RLC-16 cells.
2 MATERIALS AND METHODS
2.1 Reagents
CCl4 was purchased from Nacalai Tesque. Brefeldin A (BFA) was purchased from Merck. The following primary antibodies were used: Sec13 (1:500; Santa Cruz), Golgi58K (1:100; Sigma-Aldrich), GRP78 (1:1000; Sigma-Aldrich), beta-actin (1:10,000; Sigma-Aldrich), beta-COP (1:200; Affinity BioReagents), and calnexin (1:100 and 1:1000; Sigma-Aldrich). The Sar1 antibody (1:1000) used in the present study was previously developed in our laboratory (Nakagawa et al., 2011). The following secondary antibodies were used: horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin G (IgG; 1:2000), HRP-conjugated anti-rabbit IgG (1:1000), Cy2-conjugated anti-rabbit IgG (1:100), and Cy3-conjugated anti-mouse IgG (1:100; all from Jackson ImmunoResearch).
2.2 Animals
Animal experiments were conducted with the approval of the animal research control committee and in accordance with the guidelines for animal experiments of Osaka Prefecture University. Eight-week-old male Sprague Dawley rats (Slc: SD) were obtained from Japan SLC (Hamamatsu) and acclimatized for at least 1 week. The animals were kept in an air-conditioned room that was maintained at 22 ± 1°C and 60 ± 5% relative humidity on a 12-h light/dark cycle and were allowed free access to solid chow (CE-2; Clea Japan) and tap water.
2.3 Cell culture
The normal rat hepatocyte cell line RLC-16 (RCB1474; RIKEN Cell Bank) was cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. CCl4 was dissolved in an equal volume of dimethyl sulfoxide and then added to the culture medium for 24 h.
2.4 Cell viability
Cell number was measured using the MTT assay (Nacalai Tesque). Cell viability was measured using a trypan blue exclusion test.
The number of viable cells was determined by measuring the reduction of soluble MTT to water insoluble formazan, which is based on the reduction of a yellow tetrazolium salt (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide: MTT) to purple formazan crystals by metabolically active cells. RLC-16 cells were seeded at a density of 5 × 104/ml in a volume of 500 µl in 24-well plates and cultured for 24 h before use in the MTT assay. Cells were treated with 0.3, 1, or 3 mM CCl4 for 24 h. Before the end of incubation, 0.5 mg/ml MTT was added to each well and the cells were incubated for an 3 h at 37°C. A mixture of HCl and 2-propanol (1:9) was then added to each well to dissolve the formazan crystals and absorbance was read at a wavelength of 570 nm using a microplate reader.
2.5 Evaluation of ER-Golgi transport in RLC-16 cells
ER-Golgi transport was evaluated using a Golgi reconstruction assay (Nakagawa et al., 2012) that assessed the reassembly of brefeidin A (BFA)-treated Golgi components in RLC-16 cells. RLC-16 cells on glass coverslips pretreated with CCl4 for 24 h were treated for 30 min with BFA (10 μg/ml). After the BFA was washed out, reassembly of the Golgi apparatus was induced in normal medium for 40 min. After methanol fixation and 0.3% Triton X-100 permeabilization, the cells were used for immunofluorescent staining.
2.5.1 Confocal laser scanning microscope observation
After fixation, the cells were incubated overnight at 4°C with an anti-Golgi58K mouse monoclonal antibody (1:100) and anti-calnexin rabbit polyclonal antibody (1:100) in phosphate-buffered saline containing 0.3% Triton X-100 and 0.1% bovine serum albumin and then for 2 h at room temperature with a Cy2-conjugated anti-rabbit IgG secondary antibody (1:100) and Cy3-conjugated anti-mouse IgG secondary antibody (1:100). Colocalization of cis-Golgi marker protein Golgi58K and ER marker protein calnexin was observed under confocal laser scanning microscopy (FV-3000; Olympus). The Golgi58K and calnexin colocalization area was calculated using ImageJ software (National Institute of Health).
2.5.2 Fluorescence microscope observation
After fixation, the cells were incubated overnight at 4°C with an anti-beta-COP antibody (1:200) or anti-Golgi58K antibody (1:100) in phosphate-buffered saline containing 0.3% Triton X-100 and 0.1% bovine serum albumin and then for 2 h at room temperature with a Cy2 or Cy3-conjugated secondary antibody (1:100). Architectural changes in the Golgi apparatus were observed using fluorescence microscopy (80iD-RFL; Nikon) and evaluated according to the classification originally proposed by Sonoda et al. (2007): Grade 1 (intact), with most Golgi structures concentrated at a part of perinuclear region and with a complex structure; Grade 2 (intermediate), with partially dispersed Golgi structures loosely concentrated at the perinuclear region; and Grade 3 (dispersed), with most Golgi structures fragmented. More than 200 cells in each drug treatment group were evaluated and scored using this classification to determine the morphological changes in the Golgi apparatus.
2.6 COPII recruitment assay in a cell-free system
COPII coat protein recruitment assays were performed in a cell-free system, as previously described (Aridor & Balch, 2000) but with modifications. Briefly, the system consisted of rat liver cytosol (source of Sar1 and COPII coat protein) and ER membrane fraction derived from RLC-16 cells in a reaction mixture. ER membranes (40 μg) derived from CCl4-treated (24 h) or nontreated RLC-16 cells were incubated with rat liver cytosol (200 μg) in a final reaction buffer volume of 60 μl (36 mM Hepes-KOH [pH 7.2], 70 mM KOAc, 2.5 mM MgOAc, 250 mM sorbitol, 1.8 mM CaCl2, 1.5 mM EGTA, 1 mM PMSF, 2 μg leupeptin, and 100 μM GDP) in the presence or absence of 100 μM GTPγS for Sar1 activation. After being incubated at 32°C for 15 min, the reaction samples were layered on a 15% sucrose cushion (180 μl) and were centrifuged at 16,000g and 4°C for 15 min. The ER membrane-containing pellet was analyzed using quantitative western blotting. The amounts of ER membrane-bound proteins (Sar1 and Sec13) were quantified based on densitometry of the respective protein-positive bands using Multi Gauge software (Fujifilm). The density values of the positive protein bands were normalized to that of calnexin, an ER membrane marker protein, in the corresponding lane.
2.7 Whole cell lysate preparation
Whole cell lysates were prepared as previously described (Nakagawa et al., 2011). Whole cell lysates of CCl4-treated RLC-16 cells (24 h) were solubilized in Laemmli SDS sample buffer and analyzed using western blotting with the above-mentioned antibodies against GRP78 and actin.
2.8 Statistical analysis
Data represent mean ± SD of at least three independent experiments for each experimental condition. One-way analysis of variance followed by Tukey's post hoc test was performed to calculate p values. The p < .05 was considered statistically significant.
3 RESULTS
3.1 CCl4 inhibits the proliferation and viability of RLC-16 cells
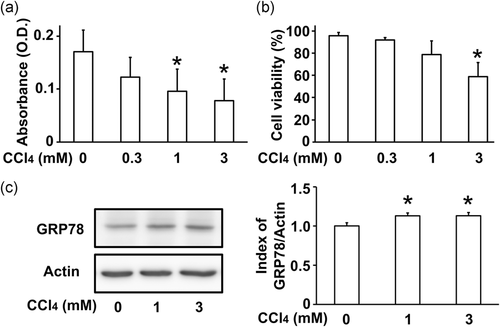
To test the cytotoxic effect of CCl4 on the RLC-16 rat hepatocyte cell line, an MTT assay was used to measure the number of viable cells and a trypan blue exclusion test to measure cell viability. The absorbance values of the MTT assay were significantly decreased in 1 mM and 3 mM CCl4-treated cells (Figure 1a). Cell viability was significantly reduced in 3 mM CCl4-treated cells (Figure 1b). Inhibition of ER-Golgi vesicle transport induces ER stress (Hikiji et al., 2015). To investigate whether the CCl4-induced cytotoxic effect is accompanied by ER stress, he protein levels of the ER stress marker GRP78 was measured using western blotting. GRP78 protein levels were slightly increased in cells treated with 1 mM and 3 mM CCl4 (Figure 1c). These results indicate that CCl4 inhibits cell proliferation and causes ER stress in RLC-16 cells.
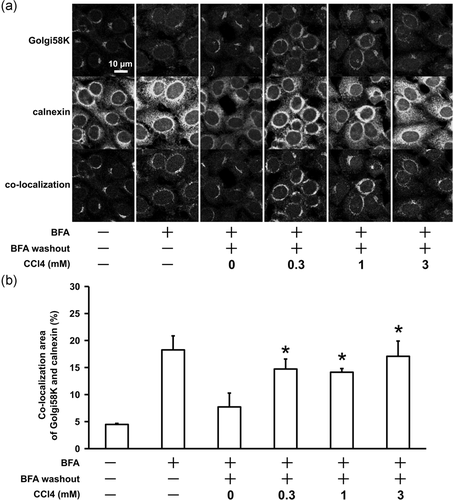
3.2 CCl4 suppresses ER-Golgi transport in RLC-16 cells
To investigate whether CCl4 inhibits ER-Golgi transport, a Golgi reconstruction assay was performed in RLC-16 cells (Nakagawa et al., 2012). BFA induced dispersion of Golgi components and their relocation to the ER (Alcalde et al., 1992). After BFA treatment, withdrawal of BFA resulted in reconstruction of the Golgi apparatus via ER-Golgi transport (Lippincott-Schwartz et al., 1989). Thus, Golgi reconstruction was evaluated after BFA washout to measure ER-Golgi transport using by confocal images of Golgi58K and calnexin double-immunostaining in RLC-16 cells. BFA treatment for 30 min induced dispersion of the Golgi components, and increased colocalization of Golgi marker (Golgi-58K) and ER marker (Calnexin). Forty minutes after BFA washout, the morphology of the Golgi apparatus returned and the colocalization of Golgi and ER decreased. Preincubation with 0.3, 1, or 3 mM CCl4 for 24 h significantly suppressed this recovery process (Figure 2).
Next, the recovery process of the Golgi apparatus was evaluated based on morphological classification of the Golgi apparatus. Architectural changes in the Golgi apparatus were observed using anti-Golgi58K immunofluorescence microscopy and evaluated according to the classification: Grade 1 (intact), with most Golgi structures concentrated at a part of perinuclear region and with a complex structure, similar to that observed under basal conditions in wild-type cells; Grade 2 (intermediate), with partially dispersed Golgi structures loosely concentrated at the perinuclear region; and Grade 3 (dispersed), with most Golgi structures fragmented and exhibiting small punctate patterns throughout the cytoplasm and spread evenly and weakly all around the nucleus (Sonoda et al., 2007). BFA treatment for 30 min induced dispersion of the Golgi components, resulting in more than 90% of the cells being scored as Grade 3. At the end of the recovery phase, 40 min after BFA washout, approximately 90% of cells had returned to the normal score of Grade 1. Preincubation with 1 and 3 mM CCl4 for 24 h retarded the recovery process, suggesting that CCl4 inhibited ER-Golgi transport (Figure 3). In addition, Golgi reconstruction assay was performed by immunofluorescence staining with another Golgi marker, beta-COP and the same results as in Figure 3 were obtained (Figure 4).
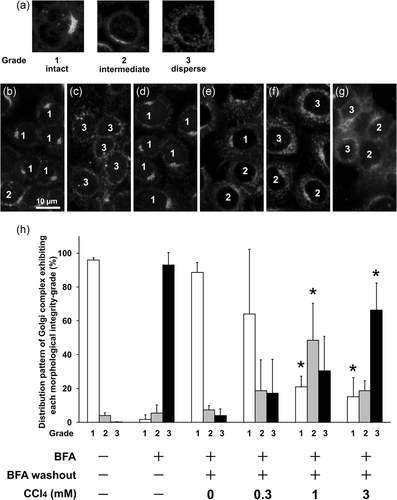
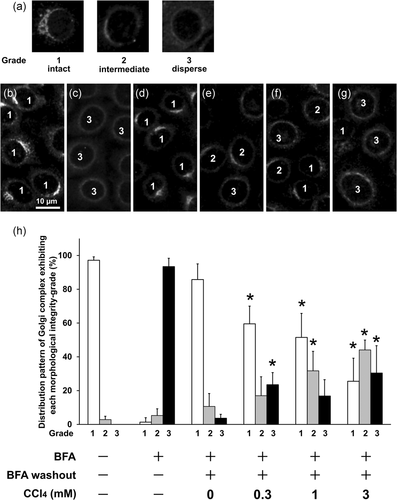
3.3 CCl4 inhibits COPII coat protein recruitment from the cytosol to the ER membrane
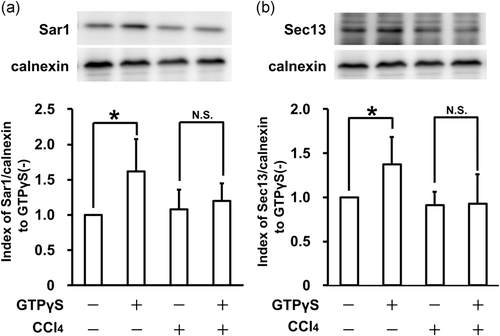
To further explore the inhibitory mechanisms of ER-Golgi transport, we focused on COPII vesicle formation on the ER membrane. To determine whether CCl4 treatment is involved in COPII coat protein recruitment, ER membrane derived from RLC-16 cells treated with 1 mM CCl4 was used in COPII recruitment assays. The translocation of COPII protein to the ER membrane was quantitatively analyzed using western blotting of Sar1 and Sec13. Addition of GTPγS increased both Sar1 and Sec13 translocation in the groups containing ER membrane derived from untreated RLC-16 cells. However, the groups using ER membrane derived from CCl4-treated RLC-16 cells showed inhibited Sar1 and Sec13 translocation (Figure 5). These results suggest that CCl4 treatment suppresses Sar1 and COPII coat protein recruitment from the cytosol to the ER membrane during COPII vesicle formation.
4 DISCUSSION
Synthesized lipids are processed in the ER to triacylglycerols. Newly synthesized triacylglycerols are packaged into vesicles and exit the ER to the Golgi apparatus for further processing (Iqbal & Hussain, 2009). In the present study, CCl4 treatment inhibited ER-Golgi transport in RLC-16 cells, suggesting that ER-Golgi transport of lipids and proteins is inhibited as part of the liver injury caused by CCl4 (Figure 2).
COPII vesicle transport is primarily mediated by Sar1 and COPII coat proteins (Sec23, Sec24, Sec13, and Sec31; Aridor et al., 1998; Bannykh et al., 1996). Mutation of Sar1, which is the initiator of COPII coat assembly, affects lipid metabolism (Chen et al., 2016). Because Anderson disease/chylomicron retention disease is caused by a Sar1 mutation, inhibition of COPII vesicle transport is known to suppress intracellular lipid transport (Georges et al., 2011; Simone et al., 2019). In patients with Anderson disease/chylomicron retention disease, Sar1 mutation reduces the GTPase activity of Sar1, suggesting that Sar1 inactivity leads to COPII vesicle transport of lipids and proteins from the ER to the Golgi apparatus (Fryer et al., 2014). Due to the inactivity of Sar1, disassembly of COPII-coated vesicles inhibits COPII transport and causes the retention of synthesized lipid in the ER lumen (Sané et al., 2017).
One question is whether lipid transport vesicles are transported by the same mechanism as protein transport vesicles. Prechylomicron assembly occurs in the ER lumen and is then transported to the cis-Golgi in prechylomicron transport vesicles (PCTVs; Mansbach & Gorelick, 2007). Compared with vesicles that carry nascent proteins, PCTVs are often large (<250 nm), with PCTVs containing Sar1 and COPII coat proteins playing a role in the export of PCTVs from the ER to the cis-Golgi (Santos et al., 2016). Fatty acid-binding protein FABP1 alters the kinetics of Sar1 GTPase activity, allowing for the secretion of large cargoes for lipids (Melville et al., 2019). Taken together, these findings suggest that Sar1 and COPII coat proteins (Sec23, Sec24, Sec13, and Sec31) are needed to form lipid transport vesicles.
It remains unclear how the changes induced by CCl4 in the ER membrane inhibit COPII coat protein translocation from the cytosol to the ER membrane. Our results showed that CCl4 treatment inhibited Sar1 recruitment and Sec13 recruitment from the cytosol to ER membranes derived from RLC-16 cells (Figure 5). The Sar1 used in this experiment had no mutation and the lack of a change in GTPase activity suggests that CCl4-induced changes in the ER membrane inhibit the recruitment of COPII coat proteins from the cytosol to the ER membrane. The binding affinity of Sar1 for the ER membrane is affected by changes in the phospholipid composition of the ER membrane. For example, increased levels of acidic phospholipids in the ER membrane ameliorate the affinity of Sar1 for the ER membrane (Matsuoka et al., 1998). Although CCl4 appears to alter the phospholipid composition of the plasma membrane (Muriel & Mourelle, 1990), there are no similar reports for the ER membrane. It is thus unknown whether CCl4 directly modifies phospholipids in the ER membrane or alters proteins that regulate the phospholipid composition of the membrane. CCl4 stimulates CYP2E1 protein modification and strongly inhibits CYP2E1 activity (Choi et al., 2002). CYP activity regulates Sar1 through ubiquitination and maintains ER homeostasis (Lin et al., 2019). The effects of protein modification by CCl4 on COPII vesicle transport regulation require further study.
5 CONCLUSIONS
We found that CCl4 treatment suppresses ER-Golgi transport in RLC-16 cells. The inhibition of COPII vesicle formation by CCl4 is a result of its ability to suppress Sar1 translocation from the cytosol to the ER membrane. These results suggest that CCl4 alters the ER membrane of hepatocytes to inhibit COPII vesicle formation, thereby causing lipid retention in hepatocytes.
ACKNOWLEDGMENTS
This work was supported by the Japan Society for the Promotion of Science JSPS KAKENHI (Grant Number: 26450408, 18K06000).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in “figshare” at 10.6084/m9.figshare.13117739, 10.6084/m9.figshare.13117754, 10.6084/m9.figshare.13117757, 10.6084/m9.figshare.13117766, 10.6084/m9.figshare.13117775.




