Ethinylestradiol and its effects on the macrophages in the prostate of adult and senile gerbils
Abstract
Prenatal and neonatal exposure to estrogenic compounds, such as ethinylestradiol (EE), promotes a variety of developmental disorders, including malformations and alterations in the morphology of glands, such as the prostate gland. Therefore, the aim of this study was to evaluate the morphological effects of neonatal exposure to EE on prostatic tissue and on the identification and quantification of gerbil gland macrophages in adult and senile Mongolian gerbils. The animals were exposed to EE (10 μg/kg/day) and to the vehicle, mineral oil (100 μL) (control group) during the first 10 days of postnatal life (lactation period). Adult gerbils were euthanized at 120 days and senile gerbils at 12 months of age. Our findings permitted verification of the presence of areas with proliferative foci in the prostate glandular portions in the adult and senile animals exposed to EE. There was also an increase in macrophages in the prostate tissue of adult and senile gerbils; these cell types alter the stromal microenvironment and possibly modify the interactions between the epithelium and stroma. Neonatal exposure to EE changes the pattern of prostatic development, leading to alterations in the arrangement of cells, including macrophages, and may be related to the onset of proliferative disorders in the prostate of adult gerbils and during aging.
Abbreviations
-
- AC
-
- adults controls
-
- AR
-
- androgen receptors
-
- BPA
-
- bisphenol-A
-
- BSA
-
- bovine serum albumin
-
- DES
-
- diethylestradiol
-
- EE
-
- ethinylestradiol
-
- EEA
-
- adults exposed to EE
-
- EES
-
- senile exposed to EE
-
- FSH
-
- follicle-stimulating hormone
-
- HE
-
- hematoxylin and eosin
-
- IL-10
-
- interleukin-10
-
- iNOS
-
- nitric oxide synthase
-
- LH
-
- luteinizing hormone
-
- M1
-
- M1 macrophages
-
- M2
-
- M2 macrophages
-
- NK
-
- natural killer
-
- PAS
-
- periodic acid Schiff
-
- PBS
-
- phosphate-buffered saline
-
- PIN
-
- prostatic intraepithelial neoplasia
-
- SC
-
- senile controls
-
- SMC
-
- smooth muscle cells
-
- TAMs
-
- tumor-associated macrophages
-
- TNF-α
-
- tumor necrosis factor-α
-
- UGE
-
- urogenital sinus
Introduction
Morphogenesis of the prostate of rodents and humans occurs in a similar mode (Prins and Putz, 2008). In humans, prostate development occurs in the prenatal stage (Timms et al., 2005), while in rodents, development begins in the prenatal period and the budding of the gland occurs in the neonatal period (Prins et al., 2001; Cunha et al., 2004).
Formation of the prostatic gland begins with a subunit of the cloaca in the urogenital sinus (UGE), which is an ambisexual embryonic structure, formed by an epithelial layer derived from the endoderm, surrounded by a mesenchymal layer of mesodermal origin (Marker et al., 2003). At this stage of development, androgens act through epithelial and mesenchymal interactions to induce prostatic budding, elongation and branching of the ducts, and epithelial growth (Cunha et al., 2002). However, during aging, there is a decline in androgen levels, resulting in changes in the form, quantity, and distribution of prostate tissue elements (Pegorin de Campos et al., 2006).
Prenatal and neonatal exposure to estrogenic compounds promotes a variety of developmental disorders, including malformations and alterations in the morphophysiology of male and female reproductive organs (Schrager and Potter, 2004; Mcpherson et al., 2007; Perez et al., 2012). In addition, other studies with rodents have observed that neonatal estrogenization causes prostatic pathologies in adulthood, which include inflammation and malignant lesions (Prins et al., 2001).
Ethinylestradiol (EE-17α-ethinylestradiol) is a synthetic estrogen present in oral contraceptives, prescribed for hormonal therapies and can be present in water and food which, together contribute as important sources of early fetal exposure to this endocrine disruptor (Smithell, 1981; Thayer et al., 2001; Schrager and Potter, 2004; Aris et al., 2014). Studies with gerbils show that exposure to EE during the prenatal period increases the development of prostatic lesions in males and females in adulthood (Perez et al., 2011, 2012) and during aging (Perez et al., 2016). But, the mechanism by which EE regulates androgen activity and the effects of its exposure in adult individuals remain uncertain (Perez et al., 2011).
Male mice exposed to low doses of EE during the prenatal development period presented abnormal prostate growth in the neonatal phase (Timms et al., 2005), added to which, when their prostates were analyzed in adult life, there was an increase in prostatic androgen receptors (AR) (Thayer et al., 2001). These studies show that the effects of EE on the prostate of newborn and adult male mice are equivalent to the glandular changes caused by Bisphenol-A (BPA) and diethylestradiol (DES).
The use of Mongolian gerbils (Meriones unguiculatus) as an experimental model is increasingly frequent in scientific research in different areas of knowledge (Jeffers et al., 1984; Nolan et al., 1990). In addition, the gerbil has been shown to be a valuable model for the study of the prostate gland (Santos and Taboga 2006; Santos et al., 2007, Fochi et al., 2008) as the gland is similar to the human prostate, regarding the compactness and fusion of the lobes, which are absent in rats and mice, where the ventral, dorsal, and dorsolateral lobes are different (Price, 1963).
The glandular portions of the prostate include the stroma, which is essential for the development, maintenance, and differentiation of the epithelium. Several cells are found in this compartment that contributes to prostate homeostasis, such as fibroblasts, smooth muscle cells (SMCs), vascular endothelial cells, telocytes, and macrophages (Vilamaior et al., 2000; Corradi et al., 2013; Felisbino et al., 2019).
In addition, macrophages are involved in the hematopoietic system, play a role in tissue repair and remodeling, homeostasis, and immunity and can relate with the production of growth factors (Parham, 2011). Macrophage doesn't consist of a single cell population with biological activity and defined phenotype but rather are a diverse collection of cell types with a wide range of functional roles in homeostatic and pathological conditions (DeNardo and Ruffell, 2019; Kim et al., 2020). There are three distinct elements that regulate the diversity of these cellular activities: developmental origin, the tissue of residence, and acute microenvironmental cues (DeNardo and Ruffell, 2019).
The role of macrophage repair and homeostasis can be subverted by chronic insults and result in a causal association of these cells with pathological states (Sica et al., 2008; Fang et al., 2013; Wynn et al., 2013). Some of these cells are known as tumor-associated macrophages (TAMs), which interact with fibroblasts, mesenchymal stem cells, endothelial, and tumor cells in order to stimulate their proliferation and promote angiogenesis (Mantovani and Allavena, 2015).
A pro-tumor function has already been demonstrated for macrophages, with great importance in the development of prostate cancer. As these cells have ARs, this receptor could modulate their function in the inflammatory process, promotion of tumor development, and the establishment of metastases (Fang et al., 2013; Comito et al., 2014; Dang and Liou, 2018).
The functional phenotype of TAMs depends on signals from the tumor and these cells can promote cancer cell proliferation (Mantovani and Allavena, 2015). The tumor microenvironment may be variable in each organism and cancer tissue (Biswas and Mantovani, 2010). In addition, there can be micro-anatomical diversity of TAM function that allows these cells to express specific cytokines and mediators for each type of macrophage and even characteristics present in both types of these phagocytes, hindering the classification in a specific macrophage (Mantovani and Allavena, 2015).
Macrophages can be classified according to their immunological performance into M1 macrophages (M1) and M2 macrophages (M2), both have distinct chemokine profiles (Biswas and Mantovani, 2010; Mimura et al., 2016; DeNardo and Ruffell, 2019). M1 also classified as classic macrophages, act directly to prevent the propagation of tumor cells. For this, they induce Th1 (CD4-Th1) cells that activate CD8 cells and natural killer (NK) cells to produce several types of interleukins, particularly interleukin 1-β (IL-1β), IL-12, IL-23, and tumor necrosis factor-α (TNF-α) (Mantovani et al., 2004). Macrophages are characterized by the presence of antigens, high inflammatory activity, microbicidal, and tumoricidal activities, high secretion of IL-6, IL-12, and IL-23, proinflammatory cytokines, low IL-10 production, and expression of nitric oxide synthase (iNOS) (Mantovani et al., 2004; Murray and Wynn, 2011; Klug et al., 2013; Cardoso et al., 2015; Facina et al., 2018; DeNardo and Ruffell, 2019).
However, M2 stimulates Th2 cells (CD4-Th2) that, in turn, activate B-lymphocytes to produce specific antibodies, which have little influence on the elimination of tumor inflammatory focus (Mantovani et al., 2004; Martinez and Gordon, 2014). These macrophages have higher expression of IL-10, arginase 1, and chemokines CCL17 and CCL22 (Biswas and Mantovani, 2010). M2 may be related to repair events, tissue remodeling, and tumor progression, besides regulating the inflammatory process (Cardoso et al., 2015).
M2 macrophages may be subdivided into M2a or alternative macrophages, which are induced by IL-4 or IL-13, and M2b or type II macrophages (activated by exposure to the immune complex and Toll-like receptors [TLRs], or IL-1R) and M2c or deactivated macrophages can be induced by glucocorticoid hormones and IL-10 (Mantovani et al., 2004; Martinez and Gordon, 2014).
In general, macrophages are involved in the majority of diseases and are attractive therapeutic targets, as their function can be increased or inhibited in order to alter the evolution of a particular disease (Wynn et al., 2013). In addition, they play a crucial role in the regulation of the hypothalamic-pituitary axis and actively act on the homeostasis of the reproductive system (Cohen et al., 1997).
Therefore, it is very important to understand how exposure to EE during the neonatal period influences the development of the ventral prostate of gerbils and also to elucidate the stromal interactions in the prostate gland during adulthood and during aging. Thus, the present study aimed to evaluate the morphological effects of neonatal exposure to EE in the ventral prostate of adult and senile male gerbils and to verify the response of stroma with a special interest in the macrophages.
Materials and methods
Animals
The female and male gerbils (Meriones unguiculatus) used in this experiment were provided by and maintained in an animal house in the IBILCE/UNESP. The animals were maintained in polyethylene boxes with wood shaving substrate, under controlled light conditions, and at an average temperature of 23°C. The animals received filtered water and rodent feed ad libitum. The experiment was in accordance with the ethical principles of animal research and was approved by the Ethics Committee on Animal Use (CEUA/UNESP, protocol 061/2012).
Experimental design
In this experiment, 10 adult virgin female gerbils were used (90–120 days) and each was maintained with a male (n = 10) of the same age. After the birth of the pups, the females were divided into two experimental groups (Figure 1). Group I (EE): 1 h after the birth, five lactating females received, by gavage, 10 μg/kg/day of EE (EE-17α-ethinylestradiol; Sigma-Aldrich, St. Louis, MO, USA) diluted in 100 μL of mineral oil, from the 1st to 10th postnatal day of the pups. According to Sanches et al. (2014) the process of prostatic budding begins on the first day of postnatal life. The dose of EE used in this experiment was similar to the dose found in oral contraceptives (Thayer et al., 2001). After weaning, the males of the brood were divided into two subgroups (1) adults exposed to EE (EEA): 10 adult males were euthanized at 120 days and (2) senile exposed to EE (EES): 10 senile males were euthanized at 365 days. Group II (Control): 1 h after birth, five lactating females received, by gavage, 100 μL of mineral oil (Nujol liquid petrolate; Mantecorp), from the 1st to 10th postnatal day of the pups. After weaning, the males of the brood were divided into two subgroups (1) adult control (AC): 10 adult males were euthanized at 120 days and (2) senile control (SC): 10 senile males were euthanized at 365 days.
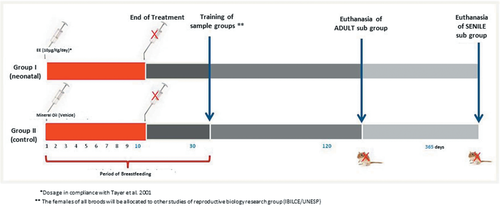
At the end of the experiment, all animals were euthanized by CO2 inhalation followed by decapitation. Body weight was measured and the prostate complex removed and dissected. After the removal of the prostate complex, the ventral lobe was separated, weighed, and fixed. The relative weight was determined through the ratio between the weight of the ventral prostate and the weight of the animal.
Morphologic, morphometric, stereologic, and karyometric analysis
The prostates were fixed by immersion in buffered 4% paraformaldehyde (pH 7.2), dehydrated in ethanol, cleared in xylene, and then embedded in Paraplast. The organs were sectioned at 4 µm and stained with hematoxylin and eosin (HE), periodic acid Schiff (PAS), and Gömöri's reticulin (Behmer et al., 1976).
Stereological analysis was carried out to determine the relative frequency of various tissue compartments (epithelium, stroma, and lumen) using the M130 multipoint test system proposed by Weibel (1978) and applied to the prostate (Huttunen et al., 1981). For this, 30 microscopic fields were captured at random from each experimental subgroup (n = 7) of the histological sections stained with HE. Morphometry was carried out to measure the epithelium height and thickness of the muscle layer. We collected 200 data points of each parameter per subgroup (n = 5) of histological sections stained with PAS. The analyses were performed using a photomicroscope Olympus BX60 and stereologic measurement using Image–Pro-Plus (Media Cybernetics, Inc., Silver Spring, MA, USA) software.
Nuclear areas and perimeters were measured in 200 nuclei of epithelial secretory cells in each group, using photomicrographs with ×1,000 magnification, of histological sections stained with HE. The form factor is a measurement of the nuclear roundness and values <1 are associated with fewer round nuclei, calculated according to the formula [=4π · nuclear area/(nuclear perimeter)2] (Taboga et al., 2003).
Statistical analyses were performed in graphics and spreadsheets of GraphPad Prism 6.00 software (GraphPad Software, Inc., CA, USA). As the normality (Kolmogorov–Smirnov test) and homoscedasticity assumptions (Levene's test) of the data appeared to be valid, data were analyzed initially by two-way analysis of variance to examine the effects of exposure to EE and age of animals as two factors. For their interaction, post hoc multiple comparisons were carried out using the Bonferroni test, and values were considered to be statistically significant when P < 0.05. Values are expressed as mean ± standard deviation.
Hormonal analysis
Blood samples were collected after decapitation, centrifuged (3,000 rpm) for 20 min, and the serum samples were frozen at −80°C for subsequent analysis. Hormonal levels were performed by capture/sandwich enzyme-linked immunosorbent assay, using specific commercial kits (Testosterone EIA Kit; Cayman Chemical Company, MI, USA). The detection limit was 6 pg/mL and readings were taken using a SpectraMax Plus 384 (Molecular Devices, CA, USA).
Immunohistochemistry
Prostate sections were subjected to immunohistochemistry (n = 7) for the detection of macrophages using primary antibodies CD68 (goat polyclonal IgG M-20; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and CD163 (rabbit polyclonal IgG M-96; Santa Cruz Biotechnology). The sections were subjected to antigen retrieval in citrate buffer (pH 6.0) at 100°C for 45 min. Subsequently, the sections were followed by three washes for 5 min in phosphate-buffered saline (PBS). H2O2 in methanol was used for 15 min to block endogenous peroxidase. Next, the sections were blocked with non-specific protein (milk powder diluted in PBS) for 30 min and then incubated with antibodies diluted in 1% bovine serum albumin (BSA) in PBS overnight (4°C, 1:50). After this, the sections were followed by three washes for 5 min in (PBS) and incubated with secondary antibody (Rabbit ABC Staining System SC-2018; Santa Cruz Biotechnology) at 37°C for 45 min, after which they were reacted with diaminobenzene (DAB; Sigma) and counterstained with Harris's hematoxylin. Negative controls were obtained by omitting the incubation with the primary antibody. The histological sections were analyzed with an Olympus BX60 light microscope.
Results
Biometric analysis
In relation to the biometric analysis, a significant increase was observed in the body weight of the males of the SC group compared with the AC and EEA and a decrease in the body weight of the EES compared with the SC. However, no statistically significant difference was found between the relative weight of the ventral prostate in the experimental groups (Table 1).
| Experimental groups | ||||
|---|---|---|---|---|
| Parameters | AC | EEA | SC | EES |
| Biometric data (n = 6) | ||||
| Body weight (g) | 60.33 ± 2.658 | 71.00 ± 5.762a | 92.33 ± 9.913b | 65.60 ± 9.363b |
| Ventral lobe relative weight (×10−4) | 2.419 ± 0.627 | 2.853 ± 1.393 | 3.22 ± 0.479 | 3.984 ± 1.349 |
| Hormonal data (%) (n = 7) | ||||
| Testosterone (ng/mL) | 2.250 ± 0.901 | 1.538 ± 0.538 | 1.369 ± 0.360 | 1.595 ± 0.468 |
- Values expressed as mean ± standard deviation. The letters are significant differences compared with (a) AC group and (b) to EEA group.
Analysis of hormonal serum levels
Testosterone test data were not significant in the experimental group of animals. However, there was a drop in testosterone levels in adult males and an apparent increase in these levels in senile males, although not significant (Table 1).
Histological analysis
Through the histological analysis of the gerbil prostates, it was possible to observe that the ventral lobe is formed by a group of acini surrounded by a fibromuscular stroma. The simple prismatic epithelium presents basal, secretory, and lumen cells filled by prostatic fluid (Figure 2). The stroma (Figure 2C) is composed of layer of SMC (Figure 2F), fibroblasts, elastic fibers, and collagen.
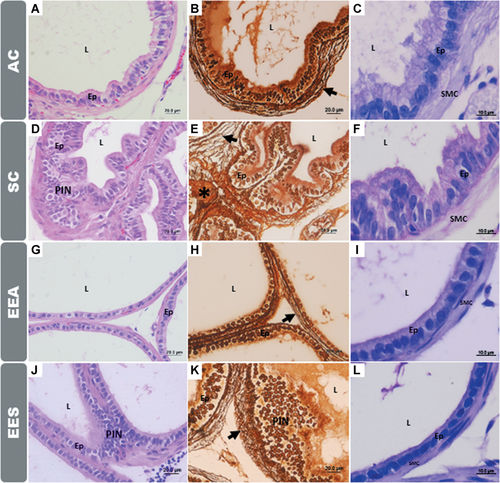
The stromal organization can also be observed by the analysis of the reticular and collagen fibers, Figures 2B, 2E, 2G, and 2K. In the EEA and EES groups, a breakdown in the morphology of the reticular fibers and an apparent increase in the collagen fibers near the basement membrane of the prostatic epithelium were observed, Figures 2E and 2K.
Exposure to EE promoted alterations in the morphology of the different tissues that make up the prostate, causing proliferative disorders that manifested in the adult and senile animals, such as the presence of regions with prostatic intraepithelial neoplasia (PINs) (Figure 2).
Stereological, morphometric, and karyometric analysis
Stereology allowed observation of alterations in the relative proportion of the tissue compartments (epithelium, stroma, and lumen) when the control and EE groups were compared. The epithelial compartment of the prostate in the EEA group increased compared with all other groups, in contrast to the senile groups in relation to CA and EEA (Figure 3A). In the stromal compartments, an increase in absolute volume was observed in the EEA group compared with senile animals (Figure 3B). However, in the prostatic luminal compartment, a decrease in the EEA group was verified, compared with all other groups and an increase in senile animals in relation to adult animals (Figure 3C).
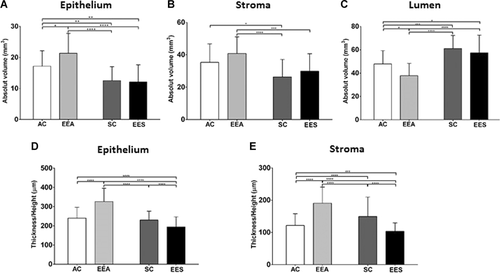
Regarding the morphometric analysis of the thickness of the epithelium and SMC of the experimental groups, in the EEA group, a significant increase in the thickness of the epithelium and SMC was observed in relation to all other groups (Figures 3D and 3E). In the prostate of the males of the EES group, a decrease in the epithelial thickness and SMC was observed compared with the other groups (Figures 3D and 3E).
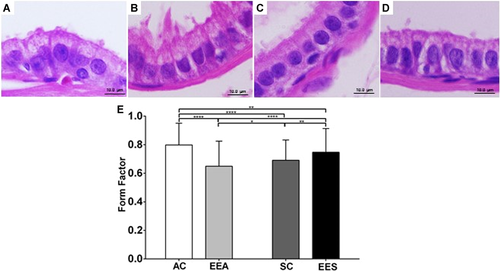
Karyometry was performed to verify the phenotypic variation found in the groups exposed to EE in relation to the controls (Figures 4A–D). The form factor was performed and verified that in the EEA group the nuclei of the epithelial cells were significantly smaller and atypical compared with the other groups (Figures 3D and 3E). In the EES group, the nuclei exhibited a round shape in relation to the EEA and SC groups (Figure 4E).
Immunohistochemical analysis
The presence of the pan-macrophages in the stromal compartments of the different experimental groups was observed with the aid of the anti-CD68 marker (Figure 5). By estimating the number of these macrophages per histological cut-off area, a significant increase in the number of these cells was observed only in the EEA group, compared with the AC group. However, the number of pan-macrophages/area was lower in the prostatic stroma of the EES group, compared with the EEA group (Figure 5).
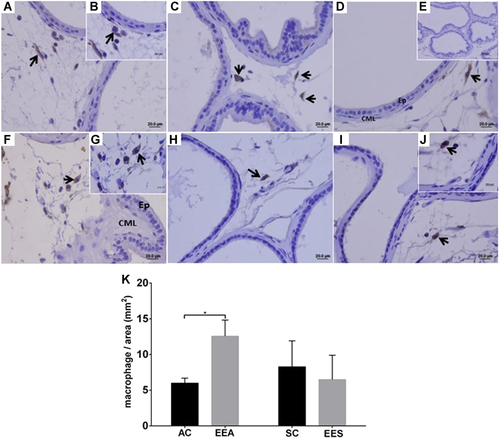
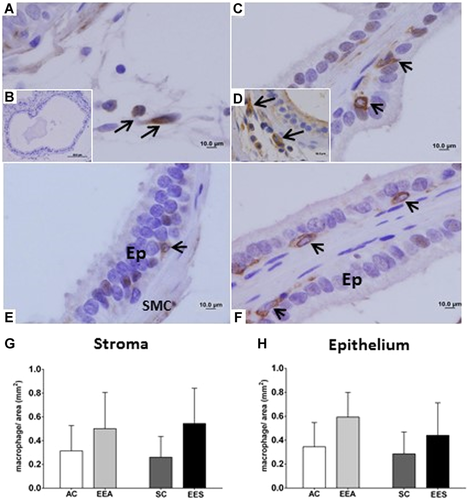
The anti-CD163 marker showed the presence of macrophages in the stroma and prostatic intraepithelial region (Figure 6). The CD163-positive macrophages presented morphological variation, with a more irregular and dendritic shape, and abnormal distribution of these cells in the prostatic regions, with the presence of proliferative foci (Figures 6D and 6E). However, in regions where the prostatic tissue did not demonstrate morphological alterations, these cells with a rounded shape were observed (Figure 6F). In the analysis of the number of CD163 macrophages/area, no significant differences were observed in the epithelial and stromal compartments; however, an apparent increase of these cells was observed in both prostatic compartments of the adult and senile animals exposed to EE during the neonatal period.
Discussion
The focus of interest in experiments involving prenatal and neonatal estrogenization of rodents (Gupta, 2000; Marker et al., 2003; Timms et al., 2005) stems from the fact that in recent decades, millions of women have exposed their children to synthetic estrogens during gestation either by intake pills, contaminated water, and food (Thayer et al., 2001; Schrager and Potter, 2004; Aris et al., 2014). To verify the effects of this exposure, the animals in the present study were exposed to EE in the lactation phase (neonatal period). This is one of the crucial phases for prostatic development in rodents and which corresponds to the development phase of the human prostate (Perez et al., 2011, 2012; Falleiros-Júnior et al., 2016; Maldarine et al., 2019; Sanches et al., 2019).
Our results allowed us to observe important morphological alterations in the ventral prostate of adult and senile gerbils, resulting from exposure to EE in the neonatal period.
Studies show that the male ventral prostate of adult and senile gerbils is more sensitive to changes promoted by exposure to EE in the prenatal period, compared with the behavior of the female prostate gland in this same exposure period (Perez et al., 2011). Unlike the female prostate gland, the male prostate gland develops in a medium with high concentrations of androgens (Santos and Taboga, 2006). The crucial events of prostatic development, such as differentiation of the urogenital system and prostatic morphogenesis, are sensitive to estrogenic action (Prins et al., 2001). This fact is evidenced by the decrease in the prostatic relative weight of an adult and senile male gerbils exposed to EE during the neonatal period.
Many studies report that the prostate gland of rodents continues to develop after birth and is complete at maturity (Marker et al., 2003; Vilamaior et al., 2006; Sanches et al., 2019). Thus, exposure to low doses of estrogenic components, such as EE, in the neonatal period may interfere with glandular homeostasis, modifying the pattern of development and, as observed in this study, promoting the development of proliferative disorders, such as PIN and changes in the organization of the stromal components, which are manifested during adulthood and during aging.
Nuclear architecture analysis is an important method to analyze cell function and pathogenesis (Gonçalves et al., 2017). These changes influence the functioning of the structural elements of the nucleus, affecting gene expression, DNA repair, and genome stability (Misteli, 2005; Gonçalves et al., 2017). Karyometry is an important factor for evaluating prostatic lesions (Taboga et al., 2003). After analysis of the nuclear form, in the present study, it is possible to infer that EE exposure influenced the shape and distribution of the epithelial cell nuclei.
Exposure to xenoestrogens (synthetic estrogens) during developmental stages is known as a risk factor for estrogen-dependent cancers, such as breast and prostate cancers, since this exposure may modify the normal tissue pattern and increase carcinogenic potential (Park et al., 2011). EE is a type of xenoestrogen and exposure to this compound during the neonatal period has led to the development of proliferative foci and morphological alterations. As in the adult male prostate, we also observed an increase in epithelial compartment volume, epithelial height, and thickness in smooth muscular layer. Neonatal exposure to estrogen is associated, in adult life, with the onset of prostatic disorders, such as inflammation and premalignant lesions (Coffey and Buchanan 2001; Prins et al., 2001; Perez et al., 2011; Falleiros-Júnior et al., 2016).
According to Atanassova et al. (1999), the hypothalamic-pituitary axis and follicle-stimulating hormone (FSH) and luteinizing hormone (LH) hormone levels in rats are extremely sensitive to neonatal estrogen exposure and may affect Leydig cell function in adulthood, such as testosterone production. In our study, although not significant, we observed changes in the testosterone levels of the experimental groups, such as a decline in these levels in the EEA group and an increase in the EES group in relation to their respective controls. A decline in testosterone levels is common during aging, a term known as late-onset hypogonadism (Wu et al., 2010). However, exposure to EE increased these levels considerably and may also be related to the decrease in the thickness of the epithelial and muscular layer of the prostate of senile gerbils.
Using the cytochemical technique of Gömöri's reticulin, it was possible to observe a disorder in the stromal compartments in the prostate of the EEA group, such as disorganization of the reticular and collagen fibers. The stromal remodeling may have been caused by an increased recruitment of macrophages, especially in the stroma, where this cell type can secrete different chemical and enzymatic mediators, actively participating in tissue remodeling (Silva et al., 2018), such as extracellular matrix metalloproteinases of types 2 and 9, as observed in prostate cancer in mice (Fang et al., 2013), in altered prostate tissue of male and female gerbils (Rochel-Maia et al., 2011) and tissue remodeling in models with hydroxyapatite grafting, or in periodontal lesions (Carneiro et al., 2009; Zambuzzi et al., 2009). According to Falleiros-Junior et al. (2016), exposure to EE during the neonatal period promotes an increase in morphological alterations, such as foci of epithelial hyperplasia associated with inflammations in the stromal and luminal compartments of the ventral prostate of adult gerbils.
Through the immunohistochemical technique, it was possible to verify the presence of macrophages in the prostate of the adult and senile animals of the control groups and those exposed to EE. These cells are part of the phagocytic system and, in adult mammals, can be found in all tissues, exhibiting great anatomical and functional diversity (Wynn et al., 2013).
Pan-macrophages are identified through anti-CD68 immunohistochemistry in regions where proliferative disorders are observed. They promote inflammatory response (Wang et al., 2014) and may contribute to the establishment of lesions such as human prostate tumors (Fang et al., 2013). CD68 is a type I transmembrane glycoprotein and is a known pan-macrophage marker (Kim et al., 2020) that can be utilized to recognize both tumoricidal M1 (Wang et al., 2020) and anti-inflammatory M2 macrophages (He et al., 2014; Barbosa et al., 2015). This may explain a higher rate of macrophages/area obtained by using the CD68 marker, which may indicate not only the presence of M1 but M2 in the prostate. The tumoricidal action of M1 can be expressed in the high levels of TNF-α, inducible iNOS, and secretion of IL-12 (Murray and Wynn, 2011; Klug et al., 2013; DeNardo and Ruffell, 2019).
In our study, in the histological sections submitted to immunohistochemistry for anti-CD163, we observed macrophages in the regions of proliferative foci of the EEA group, mainly in the areas associated with hyperplastic regions, with great diversity in their morphologies, unlike the areas with normal epithelium, where their presence was observed with a rounded shape. The resident tissue macrophages, in their basal state, present great diversity in their morphologies, transcription profiles, anatomical locations, and functional capacity (Hume, 2012; Wynn et al., 2013).
The marker CD163 is specific only to identify M2 macrophages and has been associated with several tumor growth/progression and worse prognosis (Maniecki et al., 2012; Medrek et al., 2012; Palma and Lewis, 2013; Richards et al., 2013; He et al., 2014; Barbosa et al., 2015; Kim et al., 2020). The use of only this marker suggests classifying the cells marked as M2. Therefore, despite there not being significant, the proportion of macrophages/area obtained from the use of this marker was much lower compared with the use of CD68, this indicates that CD163-positive cells are a part of pan-macrophages. These cells can express high levels of arginase 1, IL-10, CD163, CD204, or CD206 (Mantovani et al., 2002; DeNardo and Ruffell, 2019; Kim et al., 2020). These markers can be used in association to characterize M2 and changes to any of these markers permit to conclude that macrophage repolarization has occurred (DeNardo and Ruffell, 2019).
The increase in pan-macrophages in the prostate of the animals of the EEA group was statistically significant when compared with the AC group. However, in the prostate of the senile males exposed to EE during the neonatal period, a decrease in the number of these macrophages in the stromal area was observed, compared with the senile control group. Possibly, this difference between the EEA and EES groups is due to the time period between exposure to synthetic estrogen and its effects on the prostate gland. During aging, under normal conditions, the development of prostatic lesions in gerbils increases (Campos et al., 2008). Therefore, our data suggest that recruitment and macrophage activity in the prostate of adult animals may be increased as the lesions on the prostates of senile animals are established, as an attempt to prevent the appearance of these prostate lesions in adult animals exposed to EE during the neonatal period.
The exposure to EE in crucial periods of prostate development plays an important role as an endocrine disrupter, promoting changes in the hormonal receptors expression associated with prostatic disorders (Perez et al., 2011, 2016; Falleiros-Júnior et al., 2016). However, it is known that CD68 can also be a marker for TAM (He et al., 2014) and that these cells can express iNOS, IL-10, CD163, CD204, and CD206 (Mantovani et al., 2002; DeNardo and Ruffell, 2019; Kim et al., 2020), which makes it impossible to characterize it in M1 or M2 only. Besides, in our study, no changes were found that would allow inferring the presence of malignant lesions. Thus, we believe that the macrophages present in the studied prostates are not of the TAM type.
In our study, we suggest that macrophages may play crucial roles in the development of late-stage prostatic disorders, as a possible attempt to soften the effects of exposure to EE during the neonatal period. However, some questions remain to be answered with respect to the mechanisms and pathways involved in the process of tissue remodeling of the prostate gland.
Conclusion
Exposure to the endocrine disrupter EE during the neonatal period resulted in alterations in the morphology of the prostate gland that led to the establishment of proliferative disorders, altering the pattern of development and arrangement of the cells that make up the tissues of the gland during adulthood and aging. Tissue changes, particularly in the stromal remodeling, indicate that an increase in the number of pan-macrophages modifies the stromal microenvironment and, consequently, interferes with the interaction between the epithelium and stroma.
Acknowledgments and funding
The authors acknowledge the technical assistance received from MSc. Cássia Regina Suzuki Caires. The research was financed by the São Paulo State Research Foundation (FAPESP) (Scientific research grant to NFC Castro- Process Nr. 2014/05462-5 and 2012/12607-4).
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.




