Fructose-1,6-bisphosphatase degradation in the methylotrophic yeast Komagataella phaffii occurs in autophagy pathway
Abstract
Many enzymes of methanol metabolism of methylotrophic yeasts are located in peroxisomes whereas some of them have the cytosolic localization. After shift of methanol-grown cells of methylotrophic yeasts to glucose medium, a decrease in the activity of cytosolic (formaldehyde dehydrogenase, formate dehydrogenase, and fructose-1,6-bisphosphatase [FBP]) along with peroxisomal enzymes of methanol metabolism is observed. Mechanisms of inactivation of cytosolic enzymes remain unknown. To study the mechanism of FBP inactivation, the changes in its specific activity of the wild type strain GS200, the strain with the deletion of the GSS1 hexose sensor gene and strain defected in autophagy pathway SMD1163 of Komagataella phaffii with or without the addition of the MG132 (proteasome degradation inhibitor) were investigated after shift of methanol-grown cells in glucose medium. Western blot analysis showed that inactivation of FBP in GS200 occurred due to protein degradation whereas inactivation in the strains SMD1163 and gss1Δ was negligible in such conditions. The effect of the proteasome inhibitor MG132 on FBP inactivation was insignificant. To confirm FBP degradation pathway, the recombinant strains with GFP-labeled Fbp1 of K. phaffii and red fluorescent protein-labeled peroxisomes were constructed on the background of GS200 and SMD1163. The fluorescent microscopy analysis of the constructed strains was performed using the vacuolar membrane dye FM4-64. Microscopic data confirmed that Fbp1 degrades by autophagy pathway in K. phaffii. K. phaffii transformants, which express heterologous β-galactosidase under FLD promoter, have been constructed.
Abbreviations
-
- AOX
-
- alcohol oxidase
-
- bp
-
- base pair
-
- EDTA
-
- ethylenediaminetetraacetic acid
-
- FBP
-
- fructose-1,6-bisphosphatase
-
- FDH
-
- formate dehydrogenase
-
- FlDH
-
- formaldehyde dehydrogenase
-
- GFP
-
- green fluorescent protein
-
- NADP
-
- nicotinamide adenine dinucleotide phosphate
-
- PMSF
-
- phenylmethanesulfonyl fluoride
-
- PTS
-
- peroxisomal targeting signal
-
- RFP
-
- red fluorescent protein
-
- rpm
-
- revolutions per minute
-
- SD
-
- standard deviation
-
- YNB
-
- yeast nitrogen base
-
- YPD
-
- yeast extract peptone dextrose
Introduction
Methylotrophic yeasts are considered to be one of the most effective producers of intrinsic and recombinant proteins of industrial importance, in particular insulin, hepatitis B virus surface antigen, interferons, alcohol oxidase enzymes, nitrilases, and others (Gellissen, 2002; Krasovska et al., 2007; Grabek-Lejko et al., 2013). The key to creating strains, which can overproduce proteins of industrial importance, is not only the production of strains with a high level of target protein synthesis, but also the maximum reduction in the level of degradation of this recombinant protein in the cytosol. Degradation of cytosolic proteins, triggered by glucose, can occur by proteasomal degradation and/or autophagy. Some enzymes (e.g., alcohol oxidase) with peroxisomal localization have been found to be degraded by pexophagy (selective peroxisome degradation) (Tuttle and Dunn, 1995; Sibirny, 2016). Instead, the mechanisms of degradation of cytosolic proteins of their own, as well as of recombinant foreign proteins of biotechnological significance with cytosolic localization in methylotrophic yeast, remain unclear. When the yeasts are transferred from the methanol medium to the glucose-containing medium, the formation of most of the enzymes involved in methanol utilization is repressed at the transcriptional level, and the enzymes already present in the cell undergo rapid degradation and proteolysis, and the cell's carbon metabolism switches to a glycolytic pathway. This process is called catabolic degradation. Two major interacting pathways of protein degradation in eukaryotic cells have been identified: proteasomal degradation and autophagy degradation (Kirkin et al., 2009). Autophagy is a process involved in the degradation of proteins and organelles inherent in all eukaryotes (Tuttle and Dunn, 1995; Sibirny, 2011). Autophagy dysfunction is associated with cancer, neurodegeneration, microbial infection and aging (Levine and Kroemer, 2008). Therefore, the study of various aspects of autophagy on a model object of methylotrophic yeast and extrapolation of the obtained data to other eukaryotic organisms could be important for medicine. To date, 42 genes have been identified in yeast, products of which are involved in autophagy pathways (ATG genes), as well as many other genes involved, in addition to autophagy, in other related processes (Tuttle and Dunn, 1995). The balance between the processes of protein synthesis and degradation ensures the relative constancy of the protein composition of the cell. Proteasome degradation of proteins in the cell occurs in large multicatalytic protease complexes (called proteasomes) (Naujokat and Hoffmann, 2002). By selective tagging (ubiquitination) and protein degradation, the proteasome influences the cell cycle, apoptosis, proliferation, and other cellular processes, regulating the level of important signaling proteins. Fructose-1,6-bisphosphatase (Fbp1) was selected to study the possible degradation pathways of cytosolic enzymes. In glucose-containing medium, this enzyme is rapidly inactivated and degraded, making it a convenient model protein for research. Studies in baker's yeast Saccharomyces cerevisiae have shown that specific degradation of Fbp1 and malate dehydrogenase occurs with the participation of both proteasome degradation and autophagy, and also endocytosis. In addition, in baker's yeast, the pathway of inactivation of Fbp1 depends on the time of glucose starvation, in particular, in the short-term glucose starvation there is proteasome degradation of the enzyme, and in the long-term, autophagy (Hung et al., 2004; Brown et al., 2008; Menssen et al., 2012; Giardina and Chiang, 2013). The aim of this work was to investigate the ways of Fbp1 degradation in methylotrophic yeast K. phaffii.
Materials and methods
Strains and media
GS200 his4 arg4 (Waterham et al., 1996), SMD1163 (his4 pep4 prb1) (Gleeson et al., 1998), and gss1Δ (Polupanov et al., 2012) K. phaffii yeasts strains were grown in yeast extract peptone dextrose (YPD)-rich medium (1% yeast extract, 2% peptone, and 1% glucose) or YNB (0.67% yeast nitrogen base [Difco]) mineral medium with the addition of different carbon sources. Methanol (1% v/v), ethanol (1% v/v), and glucose (2% w/v) were used as carbon sources. For the growth of auxotrophic strains on mineral media, appropriate amino acids were added: l-histidine—50 mg/L, l-arginine—50 mg/L. The solid media contained agar (2% w/v). K. phaffii yeasts were grown on agar media on a Petri dish in a thermostat or cultured in liquid media with shaking of 220 rpm at 28°C. Cell biomass (in units of optical density) was determined by optical absorption of diluted suspensions by photometry on a Helios Epsilon spectrophotometer at a wavelength of 600 nm in a 1 cm wide cell.
Chemical compounds, reagents and enzymes manufactured by Sigma (USA), Fluka (Germany), Fermentas (Lithuania), and Difco (USA) were used in the work. Qualification of chemical reagents of domestic production—“hc” and “osch”.
Biochemical methods
Glass beads were used to prepare the cell-free extracts. In the first step, 50 mM Tris-HCl buffer, pH 7.5 with 1 mM phenylmethanesulfonyl fluoride was added to the precipitate washed with cell culture fluid to a final cell concentration of 50–100 mg/mL. The resulting suspension was transferred to Eppendorf plastic tubes and glass beads (diameter 0.45–0.5 mm) were added 3/4 of the suspension volume and frozen. Cells were disrupted by vortexing (vibration) for 15 min at +4°C with ice cooling every 5 min. To obtain the cell-free extract, the homogenate was centrifuged for 20 min at 14,000 rpm at +4°C on a microcentrifuge. The supernatant was used for further analysis.
Protein concentration was determined by the method described by Lowry et al. (1951) using bovine serum albumin as a standard.
To determine the specific activity of Fbp1, cells were cultivated in YNB liquid medium supplemented with 1% of methanol during 1 or 3 days (short- or long-term methanol induction). Cells were washed twice with water and shifted on the fresh YNB supplemented with 2% of glucose. Samples were collected immediately after shift from methanol to glucose (0 h), and after 6 and 24 h cultivation on glucose. Cell-free extracts were prepared. Protein concentration was determined by Lowry et al. (1951). The activity of Fbp1 was determined in a reaction mixture of the following composition (1 mL): 1 M Tris-HCl buffer, pH 8.5—100 μL; 0.5 M MgCl2—10 μL; 0.5 M ethylenediaminetetraacetic acid—2 μL; 10 mm NADP—40 μL; glucose-6-phosphate isomerase 5 μL; glucose-6-phosphate dehydrogenase—5 μL; 100 mm fructose-1,6-bisphosphate 20 μL. To start the reaction, a cell-free extract at a concentration of 0.1 mg/mL was added to the mixture and the change in extinction was detected at a wavelength of 340 nm in kinetics. This wavelength corresponds to the absorption peak of the nicotinamide cofactors. Fbp1 activity was calculated according to the equation: 1/mL of enzyme = (ΔE340/min)/(6.22 × mg of enzyme/mL of the reaction mixture), where ΔE340/min is the change of extinction at a wavelength of 340 nm per unit time (min); 6.22 is the extinction coefficient of nicotinamide cofactors.
Western blot analysis was performed using the previously described method (Baerends et al., 2000) with antibodies specific for Fbp1 and alcohol oxidase (AOX). Samples for analysis were prepared using 12.5% trichloroacetic acid and boiling with β-mercaptoethanol. Protein concentration was determined by Lowry et al. (1951) using bovine serum albumin as a standard.
Plasmids and strains construction
The K. phaffii DNA fragment encoding the FBP1 gene promoter (716 bp) was obtained by polymerase chain reaction (PCR) using a pair of primers Ko603 (CCC GAG CTC CTT ACC AGC AGC CAG GTT C)/Ko604 (CGC GAA TTC CCC GGG GCT AAT GTA AGA TAA CGT AAG AAG) and K. phaffii genomic DNA as template. The plasmid pPN6 (8,476 bp) (Polupanov et al., 2011) and the PCR product prFBP1Kp were treated with restriction enzymes SacI and EcoRI and subsequently ligated to create plasmid pPN6-prFBP1-GFP (Figure 1A).
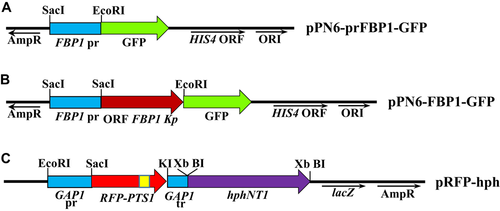
The DNA fragment encoding the FBP1 ORF (1,062 bp) was amplified by PCR using pair of primers Ko935 (TTA CGT TAT CTT ACA TTA GCA TGT CCA ATA ACA CCA CCC AAA AC)/Ko936 (CTC GCC CTT GCT CAC GAG ATT CAC AGA TTG GAT CTT AAT TTT T) and the K. phaffii genomic DNA as a template. The vector containing the FBP1 promoter fused to the GFP gene (9,192 bp) was amplified by PCR using primers Ko937 (GTG AGC AAG GGC GAG GAG CTG TTC)/Ko938 (GCT AAT GTA AGA TAA CGT AAG AAG AG) and plasmid pPN6-prFBP1-GFP, as a template. The resulting fragments were combined into single plasmid using Gibson Assembly. The resulted plasmid expressing K. phaffii FBP1 fused to GFP was designed as pPN6-FBP1-GFP (Figure 1B).
DNA fragments containing a strong constitutive promoter (481 bp) and terminator (244 bp) of K. phaffii GAP1 gene were amplified by PCR using pairs of primers Ko638 (CCG GAA TTC GTA GAA ATG TCT TGG TGT CC)/Ko673 (GAC GTT CTC GGA GGA GGC CAT GAG CTC TGT GTT TTG ATA GTT GTT CAA TTG) and Ko676 (GCC GCC ACC ACC TGT TCC TGT CCA AAT TAT AAG GTA CCA TCG ATT TGT ATG TGA AAT AGC)/Ko643 (CGC GGA TCC CAA AAG TCT TCA GGT GTT ATC) and genomic DNA of K. phaffii as a template. A DNA fragment encoding a red fluorescent protein (RFP) (729 bp) was obtained by PCR using primers Ko674 (CAA TTG AAC AAC TAT CAA AAC ACA GAG CTC ATG GCC TCC TCC GAG AAC GTC)/Ko675 (GCT ATT TCA CAT ACA AAT CGA TGG TAC CTT ATA ATT TGG ACA GGA ACA GGT GGT GGC GGC) and the pDsRed2 plasmid (Clontech) as a template. The RFP contains PTS1 sequence (serine-lysine-leucine) that provides for the transport of RFP into peroxisomes. GAP1pr, RFP, GAP1tr fragments were combined by overlapping PCR using Ko638/Ko643 primers. The obtained PCR product (1,454 bp) was treated with restriction endonucleases EcoRI and BamHI and cloned into plasmid pUC57 (Fermentas) treated with the same restriction enzymes. The hphNT1 gene (1,777 bp), conferring resistance to hygromycin was amplified by PCR using primers Ko446 (CCG GGA TCC TCT AGA GTG ATG ACG GTG AAA ACC TCT G)/Ko450 (CCG GGA TCC TCT AGA CCC AAA ACC TTC TCA AGC AAG) from plasmid pRS42H (Taxis and Knop, 2006). The obtained fragment was BamHI digested and cloned into BamHI-linearized former plasmid. The constructed plasmid was designated as pRFP-hph (Figure 1C). K. phaffii GS200 and SMD1163 (strain defected in autophagy pathway) recipient strains were transformed by the plasmid pPN6-FBP1-GFP. The introduction of DNA into yeast cells was performed by electrotransformation (Cregg et al., 1985). Prior to transformation, pPN6-FBP1-GFP was linearized with SalI restriction endonuclease. Selection of plasmid-containing transformants was performed on a mineral medium without histidine. The presence of the plasmid in the genome of transformants GS200/FBP1-GFP was confirmed by PCR (data not shown). GS200/FBP1-GFP and SMD1163/FBP1-GFP strains were transformed by HindIII-linearized pRFP-hph. Transformants were selected on solid YPD medium supplemented with 0.2 g/L of hygromycin after 3 days of incubation. The presence of the plasmid in the genome of the GS200/FBP1-GFP/RFP-PTS and SMD1163/FBP1-GFP/RFP-PTS transformants was confirmed by diagnostic PCR (data not shown). Stability of yeast transformants was determined by incubation of cells under non-selective conditions (YPD medium) for at least ten generations and subsequent analysis of the phenotype of the colonies under selective conditions and once again examined by PCR.
Microscopy
Images were taken using a fluorescence microscope (Axio Imager A1; Carl Zeiss MicroImaging, Jena, Germany) in conjunction with a monochrome digital camera (Axio Cam MRm; Carl Zeiss MicroImaging). AxioVision 4.5 (Carl Zeiss MicroImaging) and ImageJ software were used for photo processing.
Statistical analysis
All the experimental data shown in this manuscript were collected from three independent samples to ensure reproducibility of the trends and relationships observed in the cultures. Each error bar indicates the standard deviation from the mean obtained from triplicate samples. The significance level 5% were used in the statistical analyses.
Results and discussion
It is important to minimize the level of degradation of recombinant protein in the cytosol in order to achieve highest level of target proteins. We investigated the mechanisms of degradation of the cytosolic protein Fbp1 in the wild-type strain GS200 of K. phaffii, a strain with a defective vacuolar proteinase SMD1163, and a gss1Δ mutant lacking a glucose sensor. Fbp1 is a convenient model protein for the study of cytosolic protein degradation in methylotrophic yeast since it is involved in methanol utilization and its activity declines significantly and rapidly after the transfer of methylotrophic grown cells to glucose medium. Fbp1 degradation was also investigated in the presence of the proteasome inhibitor—MG132.
Changes in Fbp1 activity and its degradation by immunoblotting in the listed K. phaffii strains were studied. The impact of short (1 day) (Figures 2 and 4) and long (3 days) (Figures 3 and 4) methanol induction with subsequent shift to glucose with or without addition of proteasomal inhibitor MG132 on Fbp1 activity was investigated. The specific activity of Fbp1 in the SMD1163 and gss1Δ strains increased 2–6-fold as compared to Fbp1 activity of the wild-type strain, indicating a vacuolar degradation pathway for this enzyme. A similar effect was observed for the model peroxisomal enzyme AOX (Figures 2-4), for which a vacuolar degradation pathway was established (Tuttle and Dunn, 1995). The inactivation of Fbp1 enzyme and its degradation were found to be independent of the duration of glucose starvation. The effect of the proteаsomal inhibitor MG132 was negligible.
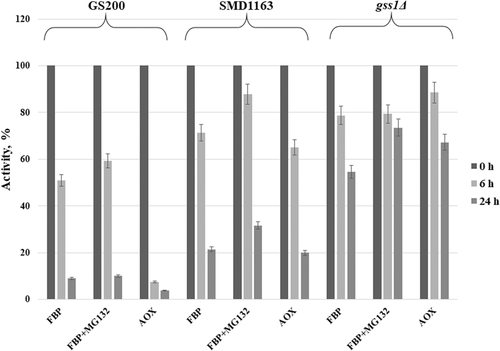
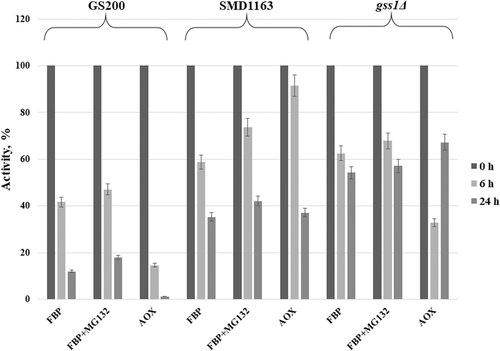
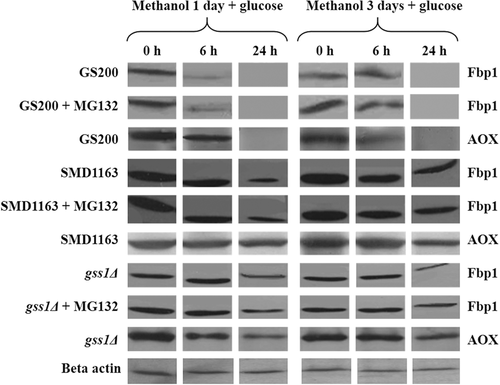
The degradation of Fbp1 was studied by western blot analysis in strains GS200, SMD1163, and gss1Δ. As shown in Figure 3, Fbp1 completely degrades on 24 h after shift of GS200 cells from methanol to glucose regardless of the duration of glucose starvation. The degradation of Fbp1 is defective in SMD1163 due to the block of vacuolar proteases. The inactivation of Fbp1 in strain gss1Δ is slower as compared with that of the wild-type strain, which is caused by a decrease in the intracellular glucose content required for catabolic inactivation of this enzyme. It can be concluded that Fbp1 degrades through a vacuolar pathway. The addition of the proteasomal inhibitor MG132 did not affect the degradation of both Fbp1 and AOX in the tested strains (Figure 4), which confirms the conclusion.
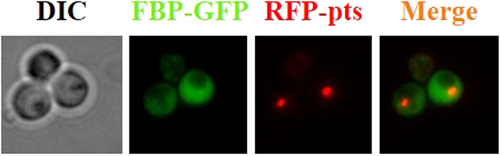
To visualize Fbp1 degradation pathway, plasmid for expression of K. phaffii FBP1 gene fused to the green fluorescent protein (GFP) and a plasmid harboring RFP tagged with a type 1 peroxisomal targeting signal (PTS1) for peroxisomes labeling were constructed.
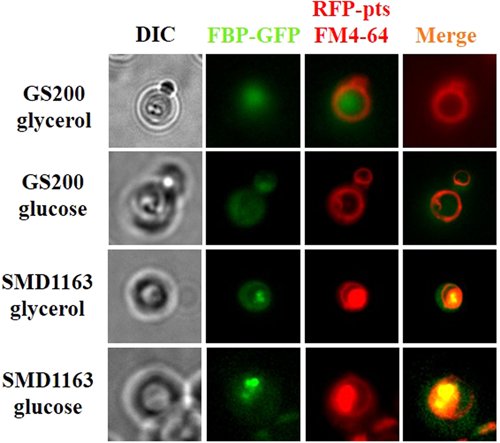
The presence of fluorescent labels in the recombinant K. phaffii strains was confirmed by fluorescent microscopy. For this purpose, recombinant and control strains were grown in minimal medium supplemented with 1.5% glycerol as a carbon source during the day at 30°C. Under the glucose starvation conditions, Fbp1 is actively synthesized and accumulated in cytoplasm of yeast cells. A green glow of the cells was observed using a green light filter. A green glow is dispersed in the cytoplasm confirming cytosolic localization of FBP1-GFP fusion (Figure 5). Under the glucose starvation red spots corresponding to RFP-labeled peroxisomes were visualized using a red filter (Figure 5). The GS200/FBP1-GFP/RFP-PTS and SMD1163/FBP1-GFP/RFP-PTS strains harboring cytosolic FBP1-GFP fusion and peroxisomal RFP were grown on glycerol medium and then shifted to glucose medium to stimulate the degradation of FBP in K. phaffii. The dye FM 4-64 was used to mark vacuolar membranes (Vida and Emr, 1995). Fluorescence microscopy images clearly demonstrated that Fbp1 in part with peroxisomes are degraded by vacuoles through autophagic pathway (Figure 6). However, degradation of Fbp1 was defective in SMD1163/FBP1-GFP/RFP-PTS with blocked vacuolar proteinases suggesting an essential role of vacuoles in degradation of Fbp1 (Figure 6). Degradation of peroxisomes in vacuoles was also strongly retarded in SMD1163/FBP1-GFP/RFP-PTS (Figure 6). Fluorescent microscopy confirmed our findings that Fbp1 is degraded by the vacuolar pathway.
Changes in Fbp1 activity and degradation of this enzyme were investigated in GS200 wild-type strain, SMD1163 autophagy-defective strain and strain with the deletion of the GSS1 K. phaffii hexose sensor gene during short- and long-term methanol induction with or without the addition of the proteasome inhibitor MG132. Obtained results of Fbp1 enzymatic assays, western blot analysis and fluorescent microscopy let us to conclude that cytosolic Fbp1 is degraded by vacuoles through autophagic pathway.
The evidence on autophagic degradation of Fbp1 in K. phaffii after shift of the methanol-grown cells to glucose raises issue on mechanisms of this process. Indeed, it is difficult to imagine how one (Fbp1) or several cytosolic proteins are recognized, sequestered and delivered to vacuole for subsequent degradation whereas most of cytosolic proteins are degraded in proteasome located in cytosol. In S. cerevisiae, Fbp1 could degrade both in proteasome and vacuole. Fbp1 delivery to vacuole occurs through so named Vid vesicles, which origin remains unknown (Hung et al., 2004; Brown et al., 2008). To study the mechanisms of cytosolic protein autophagic degradation in K. phaffii, the mutants specifically affected in this process would be important to isolate. Unfortunately, the method, based on the use of anti-Fbp1 antibodies, used for search for the mutants defective in Fbp1 degradation in S. cerevisiae, is quite laborious and expensive (Hoffman and Chiang, 1996).
Here, we decided to develop the simple method for isolation of the mutants defective in cytosolic enzyme degradation in K. phaffii using model reporter protein β-galactosidase which could be directly assayed in yeast colonies. For this, transformants of K. phaffii which express heterologous LAC4 gene from Kluyveromyces lactis coding for β-galactosidase under control of methanol-induced FLD1 promoter of K. phaffii were constructed. Resulted K. phaffii transformants expressed β-galactosidase after growth in methanol but not in glucose medium and the shift of the methanol grown cells into glucose medium led to enzyme inactivation (Figure 7). Essentially the same results were obtained after expression in K. phaffii fused gene FBP1-GFP that could be used for microscopic observation of the Fbp1 degradation (Figure 6). It was found that inactivation of β-galactosidase in transformants transferred from methanol to glucose medium is retarded in K. phaffii mutant defective in vacuolar proteinase SMD1163 whereas proteasomal inhibitor MG-132 did not influence this process both in the wild-type strain and in SMD1163 strain defective in vacuolar proteinases (Figure 7). After 24 h of incubation of methanol-grown cells with glucose, the residual level of β-galactosidase in the wild-type strain was near 24% whereas that in SMD1163 strain was 40% and addition of MG-132 did not have any effect. The reason in the observed drop of β-galactosidase activity under shift from methanol to glucose thus is neither autophagy nor proteasomal degradation. Most probably, it is result of enzyme dilution due to repression of FLD1 promoter by glucose.
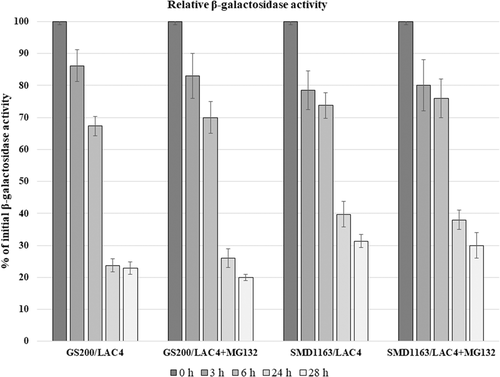
We conclude, therefore, that β-galactosidase is most probably inactivated due to degradation occurring in vacuoles, that is, by autophagy mechanism. It is important to mention that β-galactosidase of methanol-grown K. phaffii transformants can be assayed directly on plates using X-Gal staining (Figure 8), which opens opportunity to isolate the mutants defective in degradative inactivation of cytosolic proteins in K. phaffii.

Funding
This work was supported by the National Academy of Sciences of Ukraine (Grant 31-19).




