Tyrosine nitration as a key event of signal transduction that regulates functional state of the cell
Abstract
This review is dedicated to the role of nitration of proteins by tyrosine residues in physiological and pathological conditions. First of all, we analyze the biochemical evidence of peroxynitrite formation and reactions that lead to its formation, types of posttranslational modifications (PTMs) induced by reactive nitrogen species, as well as three biological pathways of tyrosine nitration. Then, we describe two possible mechanisms of protein nitration that are involved in intracellular signal transduction, as well as its interconnection with phosphorylation/dephosphorylation of tyrosine. Next part of the review is dedicated to the role of proteins nitration in different pathological conditions. In this section, special attention is devoted to the role of nitration in changes of functional properties of actin—protein that undergoes PTMs both in normal and pathological conditions. Overall, this review is devoted to the main features of protein nitration by tyrosine residue and the role of this process in intracellular signal transduction in basal and pathological conditions.
Abbreviations
-
- 3-NT
-
- 3′-nitrotyrosine
-
- NOS
-
- nitrogen oxide synthase
-
- PTMs
-
- posttranslational modifications
-
- RNS
-
- reactive nitrogen species
-
- ROS
-
- reactive oxygen species
Introduction
In academic databases, first publications on nitrative stress studies are dated 1999 and since then the number continues to grow. Today, studies concerning the effects of nitrogen oxide (NO) and superoxide are increasingly attracting the attention of biochemists, biophysicists, and physiologists. The awarding of Nobel Prize in Physiology and Medicine to American scientists R. Ferchgott, L. Ignarro, and F. Murad “for their discoveries concerning nitric oxide as a signaling molecule in the cardiovascular system” in 1998 may be the reason for such interest.
It was shown that NO and its metabolites (reactive nitrogen species, RNS) can modify amino acid residues in protein molecules, and such posttranslational modifications (PTMs) can be involved in regulation of cell signal transduction (Monteiro, 2002; Forman et al., 2003; Mikkelsen and Wardman, 2003; Schopfer et al., 2003; Thomas et al., 2004; Erusalimsky and Moncada, 2007; Matsumoto et al., 2007; Pacher et al., 2007; Bashan et al., 2009). The concept of “nitrative stress” has emerged from the understanding that protein nitration can be excessive. Under these conditions protein nitration can directly alter protein functional activity. As a result of such modifications, the cell growth is inhibited and apoptosis is activated (and/or suppressed) (van der Vliet et al., 1998; Aslan et al., 2003; Aslan et al., 2003; Reinehr et al., 2004; Ohmori and Kanayama, 2005; Salvemini et al., 2006; Pacher et al., 2007; Webster et al., 2008; Yeo et al., 2008; Yeo et al., 2008; Liaudet et al., 2009; Liu et al., 2011).
Escherichia coli possess specific “anti-nitrative system”, which includes enzymes metabolizing NO and S-nitrosothiols. In addition, proteins encoded by OxyR-controlled genes are involved in bacterial survival under both oxidative and nitrative stress (McLean et al., 2010; Poole, 2011; Vázquez-Torres, 2012). Therefore, it is suggested that eukaryotic cells have control mechanisms (in particular at the transcriptional level) similar to bacterial and such mechanisms get activated in response to oxidative and nitrative stress.
However, mechanisms that prevent the development of nitrative stress must be studied in detail. It is also important to study changes in cell's signal transduction under nitrate stress. Mechanisms of protein nitration on tyrosine residues are described in this review.
For a long period of time, proteins nitrated by tyrosine residues were considered to be “dead-end” products that would inevitably degrade. However, today there is increasing evidence of a biological denitrating system, which provides the reversibility of protein tyrosine nitration (Abello et al., 2009; Ferrer-Sueta et al., 2018). This proves the physiological role of PTM and its functions in signaling. Despite years of research, scientists have not yet given a definitive answer to question if protein nitration is a signal for degradation, or whether this modification is an important link in signal transduction. We analyze the information on the interdependence of nitration/denitration and phosphorylation/dephosphorylation of proteins. There is a large amount of data on actin nitration in normal and pathological conditions in this article.
NO role in physiological and pathological conditions
NO is highly reactive and unstable free radical. NO forms in the reaction of l-arginine oxidation to citrulline in the presence of nicotinamide adenine dinucleotide phosphate (NADPH) as electron donor. Enzymes of NO synthase (NOS) family catalyze this reaction (Davis et al., 2001; Ehab et al., 2012).
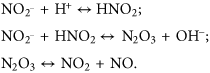
The physiological role of NO is associated with guanylate cyclase activation and increased cyclic guanosine monophosphate synthesis (Rocha Oliveira et al., 2003; Pacher et al., 2007). Other NO targets in cells are mitochondrial cytochrome c oxidase, protein kinases, phosphatases, and transcription factors that contain SH groups or metal ions in active and/or allosteric site. In particular, NO is able to regulate the activity of various members of mitogen-activating protein kinases (MAPK) superfamily, in particular p38 and JNK/SAPK (Rocha Oliveira et al., 2003; Gisone et al., 2004; Thomas et al., 2004; Cui et al., 2005; Pacher et al., 2007). In this case, NO is known to play an important role as a signaling molecule in cellular signal transduction and as a cytotoxic effector molecule of immune response.
Peroxynitrite is a key element that determines the contrasting role of NO in physiological and pathological conditions. As this free radical irreversibly modifies a large number of intracellular targets, its formation is a prerequisite for the development of nitro-oxidative stress (Pacher et al., 2007).
NO reacts with superoxide anion (О2•–) to form peroxynitrite (ООNO–) and hydroxyl radical (•OH) (Radi et al., 2001). It is proved that ONOO– formation sites are spatially linked to О2•– sources (e.g., membrane-linked NAD(P)H-oxidases, mitochondrial respiratory chain complexes) because NO is more stable and diffusible free radical than О2•– (Szabó et al., 2007; Bartesaghi and Radi, 2018). In specific cellular compartments in vivo 50–100 μM ONOO– per minute is produced (Alvarez et al., 2004).
Peroxynitrite is more reactive than its precursors, NO and superoxide (Beckman and Koppenol, 1996). Under physiological pH, a half-life of ОNОO– is extremely short (~10–3 s) (Szabó et al., 2007; Radi, 2013; Bartesaghi and Radi, 2018). Peroxynitrite is able to cross cellular or mitochondrial membrane via anion channel and immediately reacts with adjacent molecules that are located at a distance of ~5–20 μm (Szabó et al., 2007). Nevertheless, peroxynitrite reacts with the majority of biomolecules in rather low speed and with high selectivity (Pacher et al., 2007).
Peroxynitrite is a strong oxidant and nucleophile that can react with partial positively charged molecules (Pacher et al., 2007; Ferrer-Sueta and Radi, 2009; Radi, 2013). Peroxynitrite is able to oxidize ascorbic acid, thiols, modify proteins (via nitration, nitrosylation, and oxidation), lipids (via oxidation and nitration), and nucleic acids (via oxidation and nitration), as well as decompose carbohydrates (Alvarez and Radi, 2003; Gisone et al., 2004; Pacher et al., 2007; Szabó et al., 2007; Webster et al., 2008; Vandelle and Delledonne, 2011; Radi, 2013).
High levels of NO and ОNОO– induce rapid reductions in oxygen consumption, this is due to the inhibition of mitochondrial electron transport chain complexes I and IV and modification of other mitochondrial proteins (Radi et al., 2002; Turko et al., 2003; Aulak et al., 2004; Gisone et al., 2004; Koeck et al., 2004a, b; Radi, 2004, 2013; Salvemini et al., 2006; Pacher et al., 2007; Szabó et al., 2007; Berg et al., 2011; Abdelmegeed and Song, 2014). Mitochondria play an important role in peroxynitrite-mediated cell death. Exposure to high concentrations of ОNОO– often leads to rapid cell death by necrosis due to impaired energy metabolism (Pacher et al., 2007; Szabó et al., 2007). On the contrary, lower concentrations of ОNОO– lead to slow, programmed cell death by apoptosis (Beckman, 2001; Pacher et al., 2007; Szabó et al., 2007).
NO-induced modifications of molecules
It was shown earlier that NO reacts with proteins, lipids, and nucleic acids, and play an important role in signal transduction during cell development, aging, and nitro-oxidative stress.
There are several types of PTMs induced by RNS reacting with specific amino acid residues (Greenacre and Ischiropoulos, 2001; Alvarez and Radi, 2003):
Nitration—addition of a nitro group (–NO2) to organic molecules by substitution of a hydrogen atom and formation of covalent bonds. Tyrosine, tryptophan, phenylalanine, and histidine are modified in this way.
Nitrosation—reaction of nitro group (−NO) covalent attachment with the replacement of hydrogen atom from CH–, OH–, NH– groups of organic compounds. Products of the reaction are C-nitroso compounds (R–N═O), O-nitroso compounds (RO–N═O), N-nitroso compounds (R1N(–R2)–N═O), depending on which group nitro groups is attached.
Nitrosylation—addition of nitro group (–NO) to molecule due to bonding formation between metal ion and nitrogen atom. There are also nitrosyl derivatives where nitrogen of the nitroso group (–NO) is attached to non-metallic group, such as nitrosyl chloride (Cl–N═O) and S-nitroso compounds (R–S–N═O).
Protein tyrosine nitration
Tyrosine (Y, Tyr, 4-hydroxyphenylalanine) is an aromatic amino acid. Molecules of most natural proteins contain about 3% tyrosine residues among all amino acid residues. Tyrosine is a moderately hydrophilic amino acid due to the presence of a hydrophobic aromatic benzene ring that contains a hydroxyl group. As a consequence, tyrosine residues are often exposed on the protein surface (only 15 % of tyrosine residues are inside the molecule) and therefore are available for modification, in particular nitration with the formation of 3′-nitrotyrosine (3-NT) (Ahsan, 2013; Radi, 2013).
Tyrosine nitration is a covalent modification that involves the attachment of a nitro group (−NO2) to the hydroxyl group on ortho carbon of a phenolic ring (Yeo et al., 2008; Ahsan, 2013).
- –
peroxynitrite-mediated (classic) (pathway I),
- –
peroxidase-induced (enzymatic) (pathway II),
- –
metalloprotein-dependent (non-enzymatic) (pathway III).
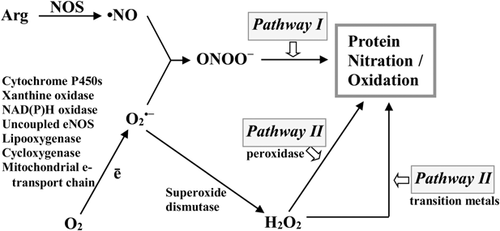
Peroxynitrite-mediated (classic) pathway
Peroxynitrite does not directly react with tyrosine residues; it must be converted to other molecules that are potent oxidizing or nitrating agents (Radi et al., 2000). Considering that the pKa of peroxynitrite anion is 6.8, ~20% of its total content is protonated to peroxynitric acid (ONOOH), which is less stable than ONOO– in physiological pH. As a result of the homolytic cleavage of peroxynitrite protonated form (ONOOH), a hydroxyl radical (•OH), and nitrogen dioxide radical (•NO2) are formed (Figure 2) (Monteiro et al., 2008; Radi, 2013; Thomson, 2015).
Proton-mediated decomposition of ONOO– is relatively slow process. Thereby ONOO– is more likely to react with Lewis acids, in particular CO2 and metal ions of heme proteins. The result of the reaction of ONOO– as nucleophile with CO2 is a formation of an intermediate adduct, nitroso-peroxycarboxylate (ONOOCO2–), which decomposes in one-electron reaction into carbonate radical (•CO3–) and nitrogen dioxide radical (•NO2) (Figure 2) (Augusto et al., 2002; Alvarez and Radi, 2003; Radi, 2013). Thus, CO2 can promote tyrosine nitration by peroxynitrite at physiological pH.
-
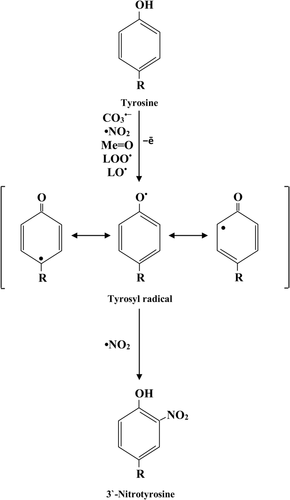
One-electron oxidation of phenolic ring with the production of tyrosyl radical (•Tyr).
-
Reaction of tyrosyl radical with •NO2 and 3-nitro-l-tyrosine formation.
Peroxidase-induced (enzymatic) pathway
Another mechanism of tyrosine nitration depends on the generation of •NO2 by various heme-containing peroxidases (mainly myeloperoxidase) in the presence of hydrogen peroxide (van der Vliet et al., 1997; Greenacre and Ischiropoulos, 2001; Brennan et al., 2002; Gaut et al., 2002).
It is considered that myeloperoxidase reacts with H2O2 to form compound I (MPO•π+(Fe4+)), which can further react with nitrite (NO2–) and produce compound II (MPO(Fe4+)) and •NO2. The last one modifies tyrosine residues (Figure 4) (van der Vliet et al., 1997; Radi, 2004).
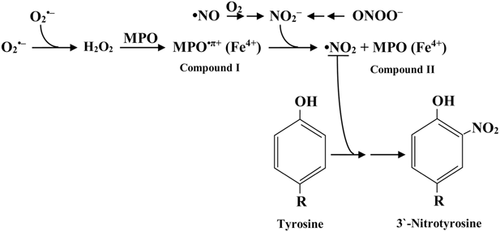
Metalloprotein-dependent (non-enzymatic) pathway
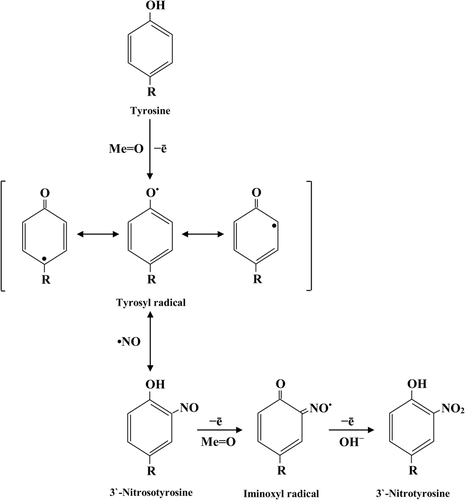
Tyrosine nitration is enhanced in the presence of transition metal ions due to the formation of •NO2 secondary radicals. Metalloproteins such as heme-containing proteins (e.g., prostacyclin synthase and cytochrome c), Cu, Zn-, or Mn-superoxide dismutase (MnSOD), and metal complexes (e.g., Mn-porphyrins) can catalyze peroxynitrite-mediated tyrosine nitration. These proteins catalyze reaction of tyrosyl radical with •NO to form 3-NT followed by a sequential two-electron oxidation to 3-NT via tyrosine iminoxyl radical (Figure 5) (Ferrer-Sueta et al., 2002; Alvarez and Radi, 2003; Alvarez et al., 2004; Radi, 2013).
Tyrosine nitration alters key physical and chemical properties of amino acids (phenol group pKa, redox potential, hydrophobicity/hydrophilicity, and molecule size). Tyrosine nitration leads to alteration in structure and functions of proteins due to changes in pKa of tyrosine hydroxyl group from 10.3 to 7.2–7.5. Reduction of pKa results in 50% tyrosine convertion to phenolate. Thereby, at physiological pH nitrated tyrosine is negatively charged. This causes changes in structure and catalytic activity of proteins in local chemical environment (Radi, 2004, 2013).
Features of nitration process of tyrosine residues under physiological condition
The nitro functional group due to its large size has a direct effect on functional properties of individual proteins. Selectivity of protein nitration, quite often even with one tyrosine residue, is sufficient enough to alter the protein's functional state (Quint et al., 2006; Tao et al., 2006; Yin et al., 2010).
In addition, tyrosine nitration can directly prevent the phosphorylation of the same residue, thereby potentially it can inhibit the functioning of phosphotyrosine-mediated signaling cascades (Gow et al., 1996; Monteiro et al., 2008). In contrast, at the cellular level it had been demonstrated that tyrosine nitration causes an increase in the total level of phosphotyrosine in the proteome (Brito et al., 1999; Aburima et al., 2010). Thus, it is obvious that this PTM is involved in ensuring the normal functioning of cells. It is confirmed by the fact that 9.27% of the total human proteome has tyrosine nitration sites (Ng et al., 2013).
Protein nitration occurs under physiological conditions, in the absence of any pathological state. Under normal conditions the amount of 3-NT varies and depends on the type of tissue or body fluid. The relatively high basal level of nitrated proteins was found in the nervous tissue, blood vessels, tissues of heart, lungs, liver, kidneys, pancreas, skeletal muscle, skin, mucous membrane of the mouth, thymus, as well as in the plasma and cerebrospinal fluid. In the vast majority of cases, the level of free 3-NT is higher (counted on molar percentage of tyrosine) than the level of 3-NT modified proteins. This may indirectly indicate that nitration of tyrosine in proteins is a more selective process. Such selectivity can be explained by the fact that individual tyrosine residues are protected from nitration by the features of the tertiary protein structure or hydrophobicity of certain domains. All those prevent access of nitrating agents to tyrosine residue.
-
Not all tyrosine residues are available for nitration. Tyrosine, which is not in direct contact with the soluble phase, is inaccessible for nitration. The secondary structure of protein and the local environment of tyrosine residue may be important determinants of nitration possibility. Almost all of the tyrosine residues that undergo nitration are localized in loop structures. There has not been any universal homologous sequence established that can be recognized by various nitrating agents. However, most nitration sites are found to contain one or more acidic residues and a relatively small amount of cysteine, methionine, and basic amino acid residues (Steinz et al., 2019). The presence of a negative charge near tyrosine residues is crucial for nitration, but the role of such negative charge in the nitration process remains unclear. It has previously been suggested that the carboxyl group of glutamate may react with peroxynitrite to form a nitro-acyl intermediate—an agent that may cause tyrosine nitration. In addition, it was suggested that the carboxylic group of acidic residues facilitates the nitration of adjacent tyrosine residues by forming hydrogen bonds with one of the two equivalent hydrogen atoms in ortho position (CE1 or CE2) of the tyrosine. Alternatively, it was suggested that a negative charge may, to some extent, direct nitration to a specific tyrosine residue by increasing the local concentration of the nitrating agent near this amino acid. Electrostatic repulsion of negatively charged nitrating agents and carboxyl groups directs them to the aromatic ring of adjacent tyrosine.
-
Different nitrating agents select different protein targets. Analysis of the amino acid sequence around tyrosine nitration sites shows that there is no apparent sequence homology that is recognized by all the nitrating agents. However, it was found that if near sites of modification are present cysteine and methionine or basic amino acids (mostly the guanidino groups of arginine) could perform as alternative targets for peroxynitrite/CO2 and HOCl/NO2−, respectively. If tyrosine residues are nearby lysine residues and cysteine (forming disulfide bonds) they do not undergo nitration. Thus, the interaction of tyrosine residues with the nitrating agents can be eliminated by steric hindrance and alternative targets (Souza et al., 1999). For example, peroxynitrite-mediated protein tyrosine nitration caused strong inhibition of MnSOD catalytic activity, while H2O2/NO2− mediated reaction resulted in less pronounced loss of activity MnSOD (Abello et al., 2009).
-
The number of tyrosine residues in proteins and the concentration of protein in solution do not affect the priority of the target protein nitration.
Under basal conditions, the largest amount of nitrated proteins at the cellular level is found in mitochondria (Bolan et al., 2000; Greenacre and Ischiropoulos, 2001). The increased level of nitration in these organelles may be related to the increased amount of peroxynitrite formed there. Under normoxic conditions (normal oxygen supply), about 0.1–3% of oxygen that are supplied to mitochondria are converted to superoxide. Instead, during hypoxia and reoxygenation the generation of superoxide increases with its peaks during transitions between hypoxic and normoxic conditions. After formation, superoxide may react with NO in a controlled reaction, which results in the formation of a highly active oxidizing agent—peroxynitrite. ONOO− in the presence of CO2/anionic bicarbonate may react with tyrosine residues with 3-nitro-l-tyrosine formation. From this it follows that a particularly high probability of protein nitration exists in mitochondria under basal conditions. This is confirmed by the fact that in mitochondria under normal conditions a significant number of proteins of important metabolic and antioxidant pathways are nitrated. In particular, it was found that enzymes of β-oxidation of fatty acids (acyl-CoA-dehydrogenase, enoyl-CoA hydratase, and β-ketothiolase), tricarboxylic acid cycle (malate dehydrogenase, aconitase, and glutamate dehydrogenase), amino acids and ketone bodies metabolism, and also proteins of respiratory chain (I and IV respiratory complexes, cytochrome c, and flavoproteins) are nitrated. Also adenosine triphosphate (ATP) synthase, anion channels dependent on the trans membrane potential, succinyl-CoA: 3-oxyacyl CoA-transferase, and Mn-SOD are nitrated (Koeck et al., 2005). A high level of nitrated proteins in mitochondria indicates the possibility of participation of this process in the regulation of cell energy supply (White et al., 2010).
Participation of protein nitration in intracellular signal transduction
The involvement of protein nitration in intracellular signal transduction, potentially, can be realized by two possible mechanisms. First one is the modification of other signaling pathways, such as tyrosine phosphorylation/dephosphorylation. Second one is the existence of a separate pathway based on the tyrosine nitration/denitration. It is possible that both of these mechanisms exist simultaneously (Koeck et al., 2005).
Interconnection of tyrosine nitration with phosphorylation/dephosphorylation of tyrosine
Tyrosine nitration causes inhibition of its phosphorylation
Tyrosine nitration causes inhibition of phosphorylation of this amino acid. In particular, it was found that in vitro peroxynitrite-mediated nitration of a single tyrosine residue in cdc2 protein, a cell cycle kinase, prevents its phosphorylation by the same amino acid residue (Kong et al., 1996). This observation has been confirmed and expanded by a group of other scientists, which shows that peroxynitrite in the bovine endothelial pulmonary artery cells causes a decrease in the level of tyrosine-phosphorylated proteins and an increase in the level of nitrotyrosine-containing proteins (Gow et al., 1996.). Another nitration target is the phosphatidylinositol-3′-kinase (PI-3′ kinase) of RAW 264.7 macrophages. Tyrosine nitration of the p85 (regulatory subunit of PI-3′ kinase) prevents its association with the catalytic p110 subunit. Suppression of the interaction between those two subunits probably disturbs the PI-3′ kinase-mediated signal transduction inside the cell, thus counteracting tyrosine nitration to its phosphorylation (Hellberg et al., 1998; Elshaer et al., 2018). All these studies allow us to suggest that tyrosine phosphorylation and nitration in proteins are mutually exclusive processes. In this case, the involvement of tyrosine nitration in cellular signaling can only be mediated by the inhibition of tyrosine residues switchover between its phosphorylated and unphosphorylated state. However, this is a rather simplistic idea of the involvement of nitration in cell signaling (Monteiro, 2002).
Tyrosine nitration enhances its phosphorylation
In several independent studies using human platelets, human erythrocyte membranes, SHSY5Y cells, and human T-lymphocytes it was shown that cells incubation with peroxynitrite causes an increase in both nitrotyrosine and phosphotyrosine levels (Mondoro et al., 1997; Mallozzi et al., 1997; Li et al., 1998; Brito et al., 1999).
The characteristics of peroxynitrite, such as short period of life and exposure at relatively low concentrations, have led to the hypothesis of peroxynitrite-mediated cell signaling. Such signaling may be mediated by tyrosine phosphorylation, and peroxynitrite acts as an inducer of such signaling events (Monteiro, 2002).
The hypothesis was further confirmed by the discovery of rapid and effective in vitro inactivation of protein tyrosine phosphatases (PTPs) by sub-micromolar concentrations of peroxynitrite. In particular, in the study of the effect of peroxynitrite on the activity of three human tyrosine phosphatases (T-cell tyrosine phosphatase CD45, the non-receptor-like tyrosine phosphatase PTP1B, and leukocyte-antigen-related phosphatase) irreversible inhibition of their activity was established. The authors claimed that complete inactivation of PTP was associated with the nitration of tyrosine residues in the protein. Peroxynitrite anion in molecular diameter and charge is structurally similar to a phosphate anion. Thus, the extreme vulnerability of PTPs to peroxynitrite-mediated inactivation is due to the attraction of peroxynitrite-anion to the enzyme active site and subsequent oxidation of thiolates. These data suggest that any PTP containing the CXXXXXR sequence in the active site can be potentially in vivo inactivated by peroxynitrite. This inactivation results in an increase in tyrosine phosphorylation and, thus, peroxynitrite affects the phosphotyrosine-dependent signaling cascades (Takakura et al., 1999).
The close relationship between nitration and phosphorylation can be confirmed by the prediction of presence of a large number of proteins that can be both phosphorylated and nitrated at the same time (Ng et al., 2013). The nitration and phosphorylation of critical tyrosine residues are competitive processes and depend on the local concentration of peroxynitrite (Brito et al., 1999). Examples of such proteins are Src kinases. In endothelial cells of the rabbit aorta, low-density lipoproteins stimulate competition between nitration and phosphorylation of the tyrosine 527 residue, which is a critical residue that regulates Src kinase activity. The presence of nitro-tyrosine 527 in the carboxy-terminal regulatory region destabilizes its closed inactive form, allowing phosphorylation of the tyrosine tyrosine 419 residue located in the kinase domain. As a result of such modifications, the Src-kinase gets activated (Monteiro, 2002).
Tyrosine nitration/denitration as a separate intracellular signal transduction pathway
As noted above, in addition to the direct regulation of tyrosine phosphorylation nitration can also be an independent signaling pathway that potentially can regulate cell function. Like any signaling pathway, the nitration/denitration pathway requires the fulfillment of four basic criteria: (I) specific target of modification, (II) modification of the activity/functionality of the modified proteins, (III) reversibility of modification, and (IV) nitration/denitration must occur during physiological period of time (Koeck et al., 2005).
Specificity of protein nitration
The specificity of protein nitration has been demonstrated both under basal conditions and in a number of tissues on models of various diseases (Aulak et al., 2001; Ischiropoulos and Beckman, 2003; Schopfer et al., 2003). The selectivity of nitration, apparently, is determined by the structure of the protein and does not depend on the nature of the nitrating agent, the relative amount of protein and the amount of tyrosine residues (Evans et al., 1996). Surface exposure of some tyrosine residues is desirable, but not required, for nitration of tyrosine residues in glutamine synthetase (Berlett et al., 1996). Regardless of the nature of the nitrating agent, some tyrosine residues have a greater propensity for nitration. The presence of tyrosine residues with a greater propensity for nitration may indirectly be an evidence of the similarity of nitration to other PTMs of tyrosine residues in proteins (sulfonation and phosphorylation) (Greenacre and Ischiropoulos, 2001).
Changes of functional properties of proteins by their nitration
Nitration of specific proteins causes changes in their functional properties (Table 1). For example, in vitro, it was demonstrated that in the presence of peroxynitrite specific nitration of the highly conserved C-terminal residue of tyrosine 363 in aldolase A occurs, with additional secondary nitration of tyrosine 342 and tyrosine 222 (Koeck et al., 2004a, b). Such nitration of tyrosine residues leads to changes in kinetic parameters (Km and Vmax), and therefore leads to the reduction of this enzyme activity. Changes in the functional properties of various proteins, including MnSOD (Comhair et al., 2005), succinyl-CoA:3-oxoacid CoA transferase (Turko et al., 2001) and actin (Schopfer et al., 2003), were also demonstrated.
| Protein | Nitration site | Functional effects |
|---|---|---|
| Actin | Tyr91, Tyr198, Tyr218, Tyr240, and Tyr296 | Impaired actin polymerization, cytoskeletal disorganization |
| Actinin | Tyr241 | Altered structure of cells (cardiomyocytes) |
| Aldolase A | Tyr174, Tyr203 | Reduced glycolytic activity |
| Alpha-synuclein | C-terminal tyrosines (i.e., Tyr125, Tyr133, and Tyr136) | Increased aggregation. Nitrated α-synuclein monomers do not aggregate but promote the aggregation of unmodified α-synuclein. |
| Apolipoprotein A-I | Tyr166 | Impaired activation of LCAT but not ABCA-1-dependent cholesterol efflux |
| Aspartate transcarbamylase | Tyr160 and Tyr213 | Decreased enzymatic activity |
| Creatine kinase | Tyr20, Tyr14, and Tyr269 | Impaired energy metabolism in the heart, skeletal muscle, and neurons |
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | Tyr92 | Reduced glycolysis, impaired energetics |
| Glutathione S-transferase (GST) | Tyr92 | Increased enzymatic-peroxidase activity |
| Heat shock protein 90 (Hsp90) | Tyr33 or Tyr56 | Toxic gain of function that led to motor neuron apoptosis through activation of the extracellular ATP receptor P2X7 |
| Tyr33 | Gain of mitochondrial regulatory function (partial inhibition of complex IV activity) | |
| Manganese superoxide dismutase (MnSOD) (SOD2) (human) | Tyr34 | Decreased enzymatic activity. Enhanced formation of peroxynitrite in mitochondria |
| Myosin heavy chain polypeptide 6 | Tyr114 and Tyr116 | Altered structure of cardiomyocytes |
| Myosin heavy chain polypeptide 7 | Tyr134 and Tyr142 | |
| Peroxiredoxin 2 | Tyr193 | A conformational change that facilitates the formation of disulfides between Prx2 monomers, an increase in peroxidase activity, and resistance to overoxidation |
| Prostaglandin endoperoxide synthase (PGHS-1) | Tyr385 | Decreased enzymatic activity |
| Protein kinase G | Tyr247 and Tyr354 | Decreased enzymatic activity. Change in cGMP binding |
| Ribonucleotide reductase R2 subunit | Three tyrosine residues found are nitrated | Decreased enzymatic activity. Inactivation did not depend exclusively on nitration of redox-active Y122 |
| SERCA | Tyr294 and Tyr295 | Reduced myocyte contractility in heart and skeletal muscle |
| Triose-phosphate isomerase (TPI) | Tyr68 | Loss of function |
| Tubulin alpha-4 chain | Tyr83 | Impaired tubulin polymerization, cytoskeletal disorganization |
| Tubulin alpha-6 chain | Tyr282 | |
| Tyrosine hydroxylase | Tyr423 | Decreased enzymatic activity, impaired neuronal dopamine synthesis |
- ATP, adenosine triphosphate; cGMP, cyclic guanosine monophosphate.
Denitration of proteins
If tyrosine nitration would be considered as a PTM, which is associated with signal transduction in cells, it must be reversible. One of the mechanisms of such posttranslational modified proteins degradation is their proteolysis. It has been demonstrated that a number of soluble proteins exposed to peroxynitrite degrade in 20S proteasomes faster than unmodified proteins (Grune et al., 1998). Nitration of tyrosine 210 in MAPK ERK1/2 initiates its ubiquitination and degradation mediated by chaperon systems and CHIP (C-terminus of Hsc70-interacting protein) (Zhang et al., 2019). Even a single nitration event is sufficient for an acceleration of target proteins degradation in proteasomes (Smith et al., 1992). It should be noted that the nitration of tyrosine residue(s) in proteins is sufficient to cause accelerated degradation of modified proteins in proteasomes and that proteasomes may be crucial for the removal of nitrated proteins in vivo (Souza et al., 2000).
However, a number of studies have found that the process of denitration is taking place in the absence of apparent proteolytic activity. A reduction of nitrotyrosine level in plasma, platelets, activated macrophages, and tissue homogenates or crude extracts from diverse organs (liver, brain, lung, heart, spleen, and prostate) at a time-, concentration-, and temperature-dependent manner was established (Abello et al., 2009). Reversibility, which involves the removal of a nitro group from tyrosine by “denitrase”, is by far the least studied criterion of nitration as a signaling pathway. Although at this stage of research the product or products of this reaction remain unknown, most researchers today are inclined to believe that there are enzymes with denitrase activity that catalyze the removal of a nitro group from nitrotyrosine in proteins. There is also some substrate specificity for the activity of such type of enzymes. Substrates of such enzymes can be nitrated bovine serum albumin and several nitrotyrosine-containing proteins, but free nitrotyrosine and some unknown endogenous nitrotyrosine-containing proteins are not its effective substrates. This substrate specificity of enzyme indicates the substrate selectivity of “nitrotyrosine denitrase” (Kamisaki et al., 1998). It should be noted that mitochondrial proteins are the ones most exposed to denitration. It indicates that mitochondria posses an organelle-specific denitration mechanism against nitrative stress. The presence of special denitration mechanism in mitochondria is due to the fact that this organelle is one of the main sources of reactive oxygen species (ROS) (Abello et al., 2009).
On the contrary, denitration can be caused by reduction of nitrotyrosine to aminotyrosine in a purely chemical reaction between Fe3+-containing heme and a reducing agent, as it can be observed in hemoglobin and myoglobin. Also a possibility of removal of the nitro group without prior reduction to aminotyrosine was established. In freshly isolated platelets, addition of calcium accelerated the denitration process with proposed reaction mechanism: Tyr-NO2 + H2O→Tyr-H + H+ + NO3− (Abello et al., 2009).
Peptides and proteins that are nitrated on tyrosine residues are not effective substrates for tyrosine kinases (Gow et al., 1996). Probably the nitration of tyrosine in the third position and the phosphorylation of the hydroxyl group in the fourth position interfere with each other. Thus, tyrosine nitration can threaten intracellular signaling via tyrosine kinase pathways, and “nitrotyrosine denitrase” activity can have a profound and important effect on cell signaling pathways. Protein denitration can restore their functional properties and allow them to become a substrate for tyrosine kinases, thereby significantly affecting the cellular signaling processes (Kamisaki et al., 1998).
Duration of nitration/denitration processes
The last criterion that the nitration/denitration processes must meet in order to be attributed to the intracellular signaling mechanisms is the period of time during which it occurs. A number of authors have shown that selective denitration and renitration in the protein occurs in the mitochondria during periodic changes of hypoxia-anoxia and reoxygenation state (Aulak et al., 2004). In highly purified rat liver mitochondria, nitration of several mitochondrial proteins decreased during 5 min hypoxia/anoxia and completely blocked after 20 min. All these changes were not accompanied by a visible loss of proteins. Nitration of the same proteins was restored within minutes after re-saturation with oxygen (Koeck et al., 2005; Yakovlev and Mikkelsen, 2010). Such experiment suggests that the process of protein nitration/denitration occurs over a physiological period of time.
Therefore, the cycle of nitration/denitration of proteins have many characteristics of the signaling pathway, including reversibility, the ability to occur during the physiological period of time, and the selectivity.
Nitration of proteins in different pathological conditions
As protein nitration is indeed an important process in the normal regulation of physiological processes, it is likely that changes in this process can lead to various pathological conditions. Cellular adaptive processes, including nitration/denitration and proteins degradation 20S by proteasomes, exist to regulate normal and slightly elevated oxidative stress. Whereas increased levels of oxidative stress can lead to excessive nitration and accumulation of nitro-modified proteins. Excessive or incorrect nitration can lead to a variety of diseases or acute pathological conditions due to dysregulation of metabolic, regulatory, and antioxidant pathways (Koeck et al., 2005).
Depending on the disease and the type of tissue, there was a 2- to 10-fold increase in the content of 3-NT modified proteins, and a 1.5–2-fold increase in nitration of free tyrosine. In some studies of rheumatoid arthritis or amyotrophic lateral sclerosis the determination of both free 3-NT and 3-NT modified protein was carried out. It was shown that, against the background of increasing free 3-NT content, there was no change in the concentration of modified proteins, which again confirms the fact that protein nitration is a selective process (Bruijn et al., 1997). Increased levels of 3-NT and 3-NT modified proteins were found in atherosclerosis (Evans et al., 1996), HIV-associated neurological complication (Bagasra et al., 1997), and spinal cord ischemia (Watanabe et al., 1996). We found the accumulation of nitrotyrosine modified proteins under the condition of diabetes (Drel and Sybirna, 2010) and low doses of irradiation (Sabadashka and Sybirna, 2016).
The increase of content of nitrated proteins in various pathological conditions was detected in the tissues of all major organs and in most cell types in vivo, including inflammatory cells (neutrophils, eosinophils, mast cells, lymphocytes, macrophages, monocytes, Kupffer cells, and astrocytes), cells of vessels (endothelial cells and smooth muscle cells), and parenchymal cells (neurons, Schwann cells, myocytes, fibroblasts, chondrocytes, hepatocytes, melanocytes, and epithelial cells). Immunohistochemical studies have shown that in the process of disease progression, protein nitration occurs in a specific cell, depending on the type of tissue or type of pathology. In some diseases, nitration occurs only in parenchymal cells, or only in inflammatory or vascular cells, but in other diseases, protein nitration occurs simultaneously in several cell types. These observations suggest that the site of formation of nitration agents can determine the type of cells and specific protein(s) that are modified by nitration. For example, O2•− formation sites may be the basis for peroxynitrite formation, while the presence of inflammatory cells ensures the availability of enzymatic catalysts, such as peroxidases (Greenacre and Ischiropoulos, 2001).
The involvement of nitration in the etiology of diabetes mellitus
In vitro, it had been shown that ONOO− could cause nitration of tyrosine in insulin receptor substrate 1 (IRS-1), thereby preventing tyrosine phosphorylation and consequently altering activation of insulin signaling (Nomiyama et al., 2004). Recent in vivo studies have suggested that nitration of tyrosine in IR, IRS, and Akt in skeletal muscle and liver may lead to impaired insulin signal transduction (Duplain et al., 2008; Zhou and Huang, 2009; Charbonneau and Marette, 2010; Elshaer et al., 2018).
In addition to the influence on insulin signaling, tyrosine nitration can disrupt the functioning of the Langerhans islands β cells. Insulin-secreting β cells of pancreatic islets, whose dysfunction is central to the pathophysiology of diabetes, are extremely sensitive to oxidative stress. Such sensitivity is based on the low activity of antioxidant enzymes, which leads to a reduced ability to detoxify excess superoxide and hydrogen peroxide. Such enzymes with reduced activity include Cu/Zn-SOD, mitochondrial Mn-SOD, catalase, and glutathione peroxidase (Tiedge et al., 1997). In addition, factors such as Mn-SOD inactivation by tyrosine nitration and decreased levels of mitochondrial NADPH and glutamate, which are crucial in the antioxidant system glutathione/glutathione peroxidase/glutathione reductase, can increase the level of oxidative stress. Increased oxidative stress leads to NO-dependent increase in the content of nitrotyrosine modified proteins (Szabo et al., 2002). Hyperglycemia also induces interleukin 1b production, іnducible nitric oxide synthase expression, and activation of neuronal NOS in pancreatic islets (Jimenez-Feltstrom et al., 2005). An increase in nitrotyrosine level also causes a decrease in glucose-stimulated insulin secretion by islet cells of patients with type 2 diabetes mellitus (Lupi et al., 2007). Thus, hyperglycemia-induced oxidative modification of proteins in β cells may be a major factor leading to dysfunction or even death of these cells.
It is also worth noting, that the main target of glucose-induced protein nitration is the glycolytic pathway in β cells. Nitration of aldolase A causes inhibition of its activity (Koeck et al., 2004a, b), which, together with the simultaneous nitration of other enzymes (glyceraldehyde 3-phosphate dehydrogenase and phosphoglycerate kinase) results in a decrease in glycolytic flux. Impaired glycolysis affects the availability of intermediates for the mitochondrial tricarboxylic acids cycle (TCA). Besides the glycolysis enzymes, such TCA enzymes as malate dehydrogenase and aconitase undergoes nitration. Decreasing the activity of TCA enzymes can slow the oxidation of glucose in the mitochondria and thus reduce the level of guanosine triphosphate formation as well as NADH and FADH2. Such changes can affect the activity of the electron transport chain and therefore the hyperpolarization of membrane and ATP synthesis. Nitration causes changes in the activity of glutamate dehydrogenase, glutamate oxaloacetate transaminase 2, as well as l-hydroxyacyl-CoA dehydrogenase, and consequently affect the metabolism of amino acids, the balance of α-ketoglutarate and the β-oxidation of fatty acids. Glucose-mediated nitration of metabolic proteins may be both part of the regulation of cell function and a major cause of impaired insulin secretion by β cells (Maechler et al., 2006; Koeck et al., 2009).
PTMs of actin by tyrosine residues
In various human pathologies, nitration of Mn-SOD, neurofilament proteins L, α-synuclein, ceruloplasmin, transferrin, α1-anti-chymotrypsin, α1-protease inhibitor, β-chain fibrinogen, c-Src tyrosine kinase has been established. In animal models of pathological conditions, nitration of tyrosinghydroxylase, neurofilament proteins L, glial fibrillar acidic protein, PGI2 synthase, and calcium ATPase of sarcoplasmic reticulum and albumin was established (Greenacre and Ischiropoulos, 2001).
One of the proteins that often undergo PTMs, both in normal and in various pathological conditions, is actin. Actin, a cytoskeletal protein contained in large quantities in all cells, is one of the main substrates for at least 17 PTMs (Terman and Kashina, 2013; García-Ortiz et al., 2017). PTMs are very dynamic and often reversible processes in result of which functional properties of proteins alter by the addition of a chemical group or other protein to its amino acid residues. With the role of actin in cell growth, movement, transport, and cell division, it is crucial to understand which actin functions are altered by different PTMs.
An analysis of the actin structure of mice showed that it contains 15 tyrosine residues and some of these residues are located in key functional areas. For example, tyrosine 53 plays a role in stabilization of the interaction of DNase I-binding loop (His40-His50) with actin subdomain 2. Fluorescein labeling of tyrosine 53 leads to blocking of actin polymerization. Tyrosine 69 is also contained in actin subdomain 2. This subdomain is involved in the interactions of actin filaments and is a site that undergoes conformational changes during ATP hydrolysis. Tyrosine 143 is located in the hydrophobic pocket of actin subdomain 1, in the region that is involved in profilin binding and is important for actin polymerization (Otterbein et al., 2001). Tyrosine 306 is located in the actin subdomain 3, which forms part of the nucleotide binding pocket (Aslan, 2012). Thus, it is possible that PTM of only one or two key tyrosine residues in actin can significantly alter its functional activity.
Functional consequences of tyrosine residue phosphorylation in actin
One of the PTMs is actin phosphorylation. The actin has at least 35 amino acid residues that can be modified by phosphorylation and this PTM can either induce or inhibit its polymerization (Table 2).
| Type of posttranslational modifications | Sites of posttranslational modifications | Consequences of posttranslational modifications |
|---|---|---|
| Phosphorylation | Tyr53, Tyr69, Tyr91, Tyr143, Tyr166, Tyr169, Tyr198, Tyr218, Tyr240, Tyr294, Tyr 306, and Tyr362 | Inhibition of actin polymerization destabilization of long actin filaments promotion of shorter actin filaments stability. Loss of stress fibrils first causing polymerization disruption and then stimulating it |
| Nitration | Tyr53, Tyr69, Tyr91, Tyr198, Tyr218, Tyr240, Tyr294, and Tyr362 | Impair actin polymerization destabilization of F-actin bundles stabilization of actin nucleus and filaments. Acceleration of filament elongation |
For example, in slime mold Dictyostelium tyrosine 53 residue phosphorylation in actin cause inhibition of its polymerization, probably due to the destruction of contact between actin subunits (Baek et al., 2008). Conversely, in the slime mold Physarum, AFK (actin fragmin kinase) the calcium-dependent enzyme phosphorylates tyrosine actin residues, leading to the elongation of actin filaments. This elongation is believed to be the result of a reduction in interactions between the flagmin and actin (Furuhasi and Hatano, 1990). In both organisms, changes caused by actin phosphorylation are associated with the response of the cytoskeleton to extracellular events (locomotion, phagocytosis, and signal transduction) and transition to a rest state.
In mammals, proteomic analyzes have shown that a number of kinases are capable to phosphorylate actin and their activity varies depending on the cell type, pathological conditions, and external stimuli. Unfortunately, most of these studies are correlational and do not clearly indicate a direct relationship between kinase activity and actin phosphorylation. For example, Ser and Tyr residues in actin are phosphorylated in response to insulin via an unknown kinase, which leads to a decrease in DNAase I binding (Terman and Kashina, 2013). Instead, activation of p21-activated kinase PAK1 (that can be activated, directly or indirectly, through its interaction with PI-3 kinase) leads to actin phosphorylation. In this case, phosphorylation was accompanied by loss of stress fibrils and thus effect on the localization of F-actin (Papakonstanti and Stournaras, 2002). Similarly, Src kinase-driven phosphorylation of tyrosine residue in actin inhibits its polymerization (Hirshman et al., 2005). Kinases that affect actin polymerization also include casein kinase I, cAMP-dependent protein kinase (PKA), calcium-dependent protein kinase, and phosphoinositide-dependent protein kinase (Shibayama et al., 1986). Those kinases can act in the opposite way first causing disruption of polymerization and then stimulating it (Ohta et al., 1987). Recently, it was also shown that tyrosine 53 dynamic phosphorylation causes destabilization of long actin filaments, promotes the stability of shorter actin filaments. Tyrosine in this passion in actin can be a target not only for phosphorylation, but also for nitration (Varland et al., 2019).
Functional consequences of tyrosine residue nitration in actin
Phosphorylation is a reverse PTM that is controlled by protein phosphatases. It has clearly been shown that nitration inhibits the activity of these phosphatases. On the basis of this, it can be assumed that actin nitration affects its functional activity directly by nitrating tyrosine residues or indirectly affecting the phosphorylation process of this protein and can often be associated with the development of a number of pathological conditions.
In particular, the involvement of nitration in the development of sickle cell disease (SCD) is well demonstrated. Using the methods of immunoprecipitation and matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry, it was found that actin is the major protein undergoing nitration in the liver and kidneys of mice with SCD, where tyrosine 91, 198, and 240 residues undergo nitration (Aslan et al., 2003). It was confirmed that the nitration of tyrosine in actin affects its polymerization properties (Table 2). Indeed, given the cooperative nature of subunit assembly, the functional consequences of tyrosine nitration of actin are significant. The addition of a negative charge in form of nitrated tyrosine 198 and 240 residues located at the “minus” end of actin leads to the formation of ionic bonds with the cationic residues located at the “plus” end of the growing filament. This interaction stabilizes both the actin nucleus and filament formation, as evidenced by the reduction of the lag phase and accelerated filament elongation. Depolymerization of nitrated actin filaments is twice as slow as that of native actin, which is due to the higher affinity of actin nitrated monomer to actin filaments. The ability of actin nitration to influence actin polymerization, binds tyrosine nitration to enhanced apoptosis of liver and kidney cells in patients with SCD (Aslan, 2012).
A significant increase in the level of actin nitration in cardiomyocytes was found on the mice model of streptozotocin-induced diabetes. The authors suggest that actin nitration may be involved in the development of diabetic cardiomyopathy (Cai et al., 2006).
Like the liver, kidney, and cardiomyocyte cells, actin nitration significantly affects the function of polymorphonuclear leukocytes. These cells play an essential role in protecting the body from pathogenic microorganisms. When the pathogen enters the body neutrophils respond to it by forming various ROS, including О2•–, hydrogen peroxide, hypochloric acid, and NO (Witko-Sarsat et al., 2000). Simultaneous formation of NO and О2•– in inflammatory sites can also lead to the formation of secondary ROS, such as peroxynitrite. This reactive oxidant plays a key role, both in the protection of the host and in the pathogenesis of inflammatory diseases (Beckman and Koppenol, 1996; Lupak et al., 2017; Brodyak et al., 2018).
Owing to the high reactive nature of peroxynitrite and its formation in close proximity to the neutrophil membrane, it is likely that this ROS may have potential cytotoxic effects or effect on neutrophil functions. In addition, as peroxynitrite has a relatively long half-life compared with other free radicals and is able to diffuse rapidly through biological membranes, proteins of membrane and cytoplasm may be potential targets of peroxynitrite. Peroxynitrite can modulate the immune response of neutrophils, mainly mediated by nitration of tyrosine residues in the proteins of these cells. It should be noted that one of the most strongly nitrated proteins contained in neutrophils exposed to peroxynitrite is a protein with a molecular weight of 45 kDa, which corresponds to the molecular weight of the actin range (Rohn et al., 1999). Nitration of tyrosine that occupies 294 position in the actin protein molecule impairs actin polymerization and destabilizes F-actin bundles (Varland et al., 2019). As actin plays one of the key roles in ensuring neutrophils functional activity (Lupak et al., 2017; Brodyak et al., 2018) it can be speculated that it may be an important functional target of nitration. Peroxynitrite was found to inhibit neutrophil actin polymerization, resulting in impaired migration, phagocytosis, and NADPH-oxidase activity. Therefore, the ability of peroxynitrite to inhibit the dynamics of actin remodeling significantly influences the actin-dependent cellular processes in phagocytic cells and can modulate their immunological functions (Clements et al., 2003).
Conclusion
Despite numerous studies, the role of tyrosine nitration at the systemic level under normal physiological conditions remains unclear. Further research is required to understand the physiological role of such PTM as nitration and elucidate its significance in pathological conditions. In particular, analysis of nitroproteome in different pathological conditions may promote development of new therapeutic approaches and search for new clinical biomarkers.
Funding
This work received no specific funding. The idea of review subject and its writing were done by the scientist of the Department of Biochemistry, Faculty of Biology, Ivan Franko National University of Lviv, Ukraine.




