Apelin and stem cells: the role played in the cardiovascular system and energy metabolism
Abstract
Apelin, a member of the adipokine family, is widely distributed in the body and exerts cytoprotective effects on many organs. Apelin isoforms are involved in different physiological processes, including regulation of the cardiovascular system, cardiac contractility, angiogenesis, and energy metabolism. Several investigations have been performed to study the effect of apelin on stem cell therapy. This review aims to summarize the literature representing the effects of apelin on stem cell properties. Furthermore, this review discusses the therapeutic potential of apelin-treated stem cells for cardiovascular diseases and demonstrates the effect of stem cells overexpressing apelin on energy metabolism. Stem cells with their unique characteristics play a crucial role in the maintenance of tissue integrity. These cells participate in tissue regeneration via multiple mechanisms. Although preclinical and clinical studies have demonstrated the therapeutic potential of stem cells in various diseases, their application in regenerative medicine has not been efficient. A number of strategies such as genetic modification or treatment of stem cells with different factors have been used to improve the efficacy of cell therapy and to increase their survival after transplantation. This article reviews the effect of apelin treatment on the efficacy of cell therapy.
Abbreviations
-
- AD-MSCs
-
- adipose tissues-derived MSCs
-
- Akt /PKB
-
- protein kinase B
-
- AMPK
-
- AMP-activated protein kinase
-
- aP2
-
- adipocyte protein 2
-
- APJ
-
- apelin receptor, putative receptor protein related to the angiotensin receptor (AT1)
-
- ASCs
-
- adult stem cells
-
- BAT
-
- brown adipose tissue
-
- Bcl-2
-
- B-cell lymphoma 2
-
- BM-MSCs
-
- bone marrow-derived MSCs
-
- BMCs
-
- bone marrow cells
-
- CVDs
-
- cardiovascular diseases
-
- EPCs
-
- endothelial progenitor cells
-
- ERKs
-
- extracellular signal-regulated kinases
-
- ESCs
-
- embryonic stem cells
-
- HbA1c
-
- hemoglobin A1c
-
- HIF-1
-
- hypoxia-inducible factors
-
- MAPK
-
- mitogen-activated protein kinase
-
- MI
-
- myocardial infarction
-
- MSCs
-
- mesenchymal stem cells
-
- p70 S6K
-
- p70S6 kinase
-
- PAD
-
- peripheral artery disease
-
- PI3K
-
- phosphatidylinositol 3-kinase
-
- PKC
-
- protein kinase C
-
- ROS
-
- reactive oxygen species
-
- SDF
-
- stromal cell-derived factor
-
- UCP1
-
- uncoupling protein 1
-
- VEGF
-
- vascular endothelial growth factor
-
- WAT
-
- white adipose tissue
Introduction
Stem cells are cells capable of differentiating into tissue-specific cells. These cells exist in different tissues and play a pivotal role in repair and regeneration after tissue injuries. On the basis of the differentiation potential, these cells can be classified into five groups: totipotent, pluripotent, multipotent, oligopotent, and unipotent. Moreover, stem cells are categorized according to their origin into three major classes: embryonic, fetal, and adult stem cells (ASCs). Embryonic stem cells (ESCs) are pluripotent cells able to differentiate into all three germ lineages. ASCs reside within different tissues and participate in tissue regeneration. Mesenchymal stem cells (MSCs), an example of ASCs, express specific surface cell markers and are multipotent (Dominici et al., 2006; Smith, 2006; Ilic and Polak, 2011; Kolios and Moodley, 2013). MSCs have various differentiation capacities and statuses related to their microenvironment and origin (Watt et al., 2013; Yang et al., 2018). These cells can be isolated from endodermal, mesodermal or ectodermal tissues (Fortier, 2005). For example, MSCs derived from bone marrow MSCs (BM-MSCs) or adipose tissues MSCs (AD-MSCs) can be obtained from mesodermal tissues (Pittenger et al., 1999; Zuk et al., 2001). These cells can differentiate into different cell lineages under specific growth factors (Schuldiner et al., 2000).
Several investigations have shown the beneficial effects of stem cells on the treatment of different diseases (Hahn et al., 2008; Larijani et al., 2012; Liu et al., 2014). These cells participate in tissue repair and regeneration by different mechanisms, including homing to damaged tissues (Cheng et al., 2008), differentiation into specialized cells (Toma et al., 2002), and their paracrine effects (Li et al., 2013; Watt et al., 2013). In spite of the recent progress made in stem cell research, some limitations affect the practical applications of stem cells in cell therapy. For example, cell therapy is costly, and after transplantation, these cells may be rejected because of the immune response (Fortier, 2005). In addition, several factors, such as ischemic conditions, can affect the efficiency of cell transplantation through the reduction of cell survival (Wang et al., 2008; Huang et al., 2010).
Numerous strategies can be used to increase the regenerative capacity and therapeutic potential of stem cells including tissue engineering and genetic modification of the cells (Huang et al., 2016). The beneficial effects of hypoxic preconditioning on cell viability have been shown by Wang and colleagues (Wang et al., 2008). It is also possible to enhance cell survival by adding growth factors and peptides, which affect stem cell properties (Hahn et al., 2008; Zeng et al., 2012).
Apelin is a regulatory peptide which exerts a protective effect on different cells and tissues. Recently, its regulatory role in various signaling pathways has been reviewed (O’Carroll et al., 2013; Kurowska et al., 2018). It is associated with metabolic disorders via its effects on lipid and glucose metabolism (Castan-Laurell et al., 2011; Hwangbo et al., 2017). It has multiple biological actions, including cardiovascular function regulation, fluid homeostasis, and angiogenesis (O’Carroll et al., 2013). In addition, the beneficial effects of apelin on the efficiency of stem cell therapy have been demonstrated (Zeng et al., 2012). Therefore, the purpose of this paper is to review some current knowledge related to the apelin effects on stem cell properties. It also presents the effects of apelin on stem cell therapy for the cardiovascular system and energy metabolism. To better understand the apelin effects on the therapeutic potential of stem cells, we briefly review the current knowledge about the effects of stem cells or apelin on the treatment of diseases. Then, the effect of apelin on stem cell therapy will be discussed.
Apelin and the APJ (apelin receptor, putative receptor protein related to the angiotensin receptor [AT1]) receptor
Apelin is a member of the adipose-secreted adipokine family (Boucher et al., 2005). Its gene is located on the X chromosome encoding a 77 amino acid preproapelin (Lee et al., 2000). Apelin-36 (apelin42-77), apelin-17 (apelin61-77), apelin-13 (apelin65-77), and pyroglutaminated apelin-13 (Pyr-apelin-13) are different isoforms of the apelin peptide (Tatemoto et al., 1998). These isoforms are different based on their binding affinity, biological potency, and tissue distribution (Kawamata et al., 2001). Apelin activates its receptor named APJ, which is a member of the G protein-coupled receptor family (Tatemoto et al., 1998). This peptide is expressed in different tissues and cells such as the heart, blood vessels, and adipocytes, implying the role of apelin in multiple physiological and pathophysiological functions (Kleinz and Davenport, 2004; Boucher et al., 2005; Kleinz and Davenport, 2005; Maguire et al., 2009). Moreover, its expression can be affected by several factors such as hormones, cytokines, fasting status, and hypoxic conditions (Boucher et al., 2005; Daviaud et al., 2006; Ronkainen et al., 2007). Apelin affects several signaling cascades such as extracellular signal-regulated kinases (ERKs) and protein kinase B (PKB), thereby contributing to different cellular processes such as migration and proliferation (Masri et al., 2004; Liu et al., 2010; Li et al., 2017). It is also involved in the regulation of the cardiovascular system and hormones (Taheri et al., 2002; Kidoya et al., 2010; Azizi et al., 2013; O’Carroll et al., 2013). Moreover, it has been demonstrated that not only has apelin signaling effects on the aging process but also its expression is affected by aging. Expression of apelin and its receptor in different tissues reduces due to the aging process. Apelin signaling down-regulation is correlated with age-related diseases and accelerates them. Administration of apelin can ameliorate these disorders (Rai et al., 2017). Oxidative stress can lead to aging-associated diseases (Liguori et al., 2018). Apelin raises the activity of antioxidant enzymes (Than et al., 2014), indicating this peptide acts as an anti-aging factor (Rai et al., 2017). Apelin also decreases the reactive oxygen species (ROS) generation and pro-oxidant enzyme expression in adipocytes, regulates glucose metabolism, and improves insulin resistance. These findings suggest that apelin may be used as a promising therapeutic strategy in the treatment of oxidative stress associated metabolic diseases (Zhu et al., 2011; Than et al., 2014). Additionally, apelin has effects on angiogenesis in hypoxic conditions through increasing the proliferation of endothelial progenitor cells (Zhang et al., 2015, 2016).
Apelin and stem cells: effect on stem cell properties
Expression of apelin/APJ receptor in stem/progenitor cells
The expression of apelin and/or its receptor in various stem/progenitor cells derived from the bone marrow, Wharton's jelly, and adipose tissue has been demonstrated (Than et al., 2012; Zeng et al., 2012; Gao et al., 2018). Li et al. (2015) showed that apelin and its receptor are expressed in BM-derived stem cells. In contrast, Gao et al. (2010) revealed that the apelin and APJ receptor expression were not detectable before cardiac differentiation in rat BM-MSCs. But, their expression increased after the induction, indicating the effect of apelin on the differentiation. This is consistent with another report showing that apelin participates in cardiac differentiation of ESCs (D’Aniello et al., 2009). The expression of apelin/APJ receptor in stem cells derived from the BM can be induced in response to hypoxic conditions. Moreover, the expression of apelin/APJ is upregulated in BM-derived mononuclear cells as a result of apelin delivery in mice. In contrast, Zeng et al. (2012) showed that APJ receptor expression in BM-MSCs is not affected by apelin-13 administration (Li et al., 2013, 2015). The exact reason for the contradictory results related to the expression of apelin and APJ receptor in stem cells from different sources is not clear. Nowadays, stem cells can be isolated from different tissues. The expression of apelin isoforms and the APJ receptor in these cells is not completely defined and needs more investigations.
The effect of apelin on stem cell proliferation
Problems related to the expansion of stem cells can limit the application of stem cells in cell therapy. The number of stem cells affects patient survival after transplantation and amplification of stem cells can also be efficient in their therapeutic capacity (Wagner et al., 2002; Fortier, 2005; Walasek et al., 2012). Several investigations have indicated the role of growth factors and small molecules in the proliferation potential of stem cells (Hoggatt et al., 2009; Penna et al., 2013). Apelin can induce different signaling cascades such as the PI3K/Akt pathway and thereby increases cell proliferation (Liu et al., 2010). The mitogenic effect of this peptide on human umbilical vein endothelial cells has also been demonstrated (Masri et al., 2004).
Several studies have shown that apelin and the APJ receptor are involved in the proliferation of stem/progenitor cells obtained from various sources. Recently, the positive effect of apelin-13 on the proliferation of BM-MSCs and the viability of AD-MSCs in hypoxic conditions have been demonstrated (Liang et al., 2016; Hou et al., 2017). Indeed, this peptide plays a significant role in the proliferation of progenitor cells under hypoxic conditions. Hypoxia upregulates the expression of hypoxia-inducible factor-1 and apelin/APJ receptor and thereby enhances the proliferation of progenitor cells via the PI3K/Akt signaling pathway (Zhang et al., 2015). Consistent with these reports, Li et al. (2014, 2015) showed that apelin-13 is involved in the hypoxia-induced proliferation of BM-MSCs. Apelin promotes the stem cell proliferation through the Akt/cyclinD1 signaling pathway. In contrast, Zeng et al. (2012) showed that the proliferation of rat BM-MSCs is not directly affected by apelin-13.
The effect of apelin on stem cell differentiation
Apelin and its receptor affect the differentiation potential of stem cells through the regulation of various cell signaling pathways. Apelin and the APJ receptor can potentiate cardiac differentiation of ESCs mediated through the ERK signaling pathway. The efficiency of differentiation increases when apelin treatment is used in combination with mesodermal differentiation factors (D’Aniello et al., 2009; Wang et al., 2012). In addition to increasing cardiac differentiation, apelin plays a role in adipocyte differentiation. The expression of apelin in preadipocytes increases during adipocyte differentiation (Boucher et al., 2005). Moreover, Than et al. (2015) showed that pyr-apelin-13 increased the expression of markers related to brown adipocyte such as uncoupling protein 1 (UCP1) in preadipocytes via Akt and the AMP-activated protein kinase (AMPK) signaling pathways. Apelin also affects the adipogenic differentiation of human BM-MSCs and upregulates UCP1 expression.
The effect of apelin on stem cell survival
There are several strategies that can be used to improve the survival of stem cells, including hypoxic preconditioning (HPC) and treatment with growth factors (Hahn et al., 2008; Wang et al., 2008; Chacko et al., 2010; Penna et al., 2013).
Apelin treatment can increase the survival of stem/progenitor cells via different mechanisms. It improves the paracrine effects of BM-MSCs via upregulation of factors related to angiogenesis such as vascular endothelial growth factor (VEGF) and thereby enhances the survival and angiogenesis potential of the cells (Hou et al., 2017). It also enhances the expression of factors related to cell survival in bone marrow cells (BMCs) (Li et al., 2013). In addition, apelin reduces apoptosis in rat BM-MSCs by preventing the mitochondria cytochrome c release and the ROS generation. Apelin exerts this effect by activation of ERK and Akt pathways (Zeng et al., 2012). However, there are some reports showing that apelin-13 increases the level of ROS in different cells (Li et al., 2011; Xie et al., 2017). Apelin also can improve the efficacy of cell therapy for peripheral artery disease (PAD). Administration of this peptide increases the survival of transplanted cells in ischemic tissues through the phosphoinositide 3-kinase (PI3K)-dependent pathway. During the hypoxia phase, this adipokine enhances protective autophagy through the AMPK signaling pathway and thereby increases the therapeutic capacity of the cells. In contrast, during hypoxia/reoxygenation (H/R), it reduces autophagic cell death via phosphorylation of AKT. Thus apelin can improve the therapeutic potential of stem cells through its effect on cell survival (Liang et al., 2016).
The effect of apelin on stem cell angiogenesis
Stem cells play an essential role in angiogenesis through their paracrine effects (Watt et al., 2013). Several factors such as HPC and growth factors can affect the angiogenic potential of stem cells. Hypoxia upregulates VEGF expression, a pro-angiogenic growth factor, in rat stem cells (Chacko et al., 2010). The upregulation of pro-angiogenic growth factors can improve the therapeutic capacity of stem cells for ischemia treatment (Toyama et al., 2009).
Apelin and the APJ receptor are expressed in different parts of the cardiovascular system (Kleinz and Davenport, 2004, 2005; Kleinz et al., 2005), and it is considered as an angiogenic factor (Kasai et al., 2004). Kidoya and Takakura (2012) summarized the effects of apelin/APJ receptor on angiogenesis and vessel development. This peptide is involved in vascular development and patterning, and its effect on the proliferation of endothelial cell lines is independent of VEGF (Cox et al., 2006). A reduction in apelin signaling inversely affects angiogenesis (Kunduzova et al., 2008). Besides the role that apelin plays in the blood vessel construction (Kidoya et al., 2008), it is involved in angiogenesis through its effect on the alignment of arteries and veins (Kidoya et al., 2015).
Numerous studies have revealed the effect of apelin on angiogenic properties of stem cells/progenitor cells. Endothelial progenitor cells (EPCs) exert a critical effect on new blood vessel formation. Apelin signaling has an effect on the EPCs proliferation under hypoxic conditions. Apelin-transfected cells have a higher proliferative capacity than the untransfected cells (Zhang et al., 2015, 2016). This peptide upregulates the angiogenic factor expression in BMCs (Li et al., 2013). The increase of VEGF expression in MSCs treated with apelin enhances the vascularization potential of stem cells. Apelin-treated MSCs can affect human umbilical vein endothelial cells to form more new vascular branches (Hou et al., 2017). Moreover, Park et al. (2016) showed that the treatment of stem cells with VEGF-coated apelin-loaded poly-(DL)-lactic-co-glycolic acid nanoparticles induced endothelial differentiation of the cells. Transfected cells result in new vessel formation. Additionally, investigation of the vessel density in the PAD model revealed that apelin-treated MSCs enhance angiogenesis more efficient than AD-MSC (Liang et al., 2016).
Apelin and stem cells: effects on cardiovascular system
Cardiovascular diseases (CVDs) are one of main causes of death in the world (Benjamin et al., 2018). There are a number of interventions to treat these diseases, and most of them are not efficient. Cell-based therapy is a potentially valuable therapeutic option to treat CVDs (Wang et al., 2012; Watt et al., 2013). Although plenty of preclinical and clinical studies have demonstrated the potential of stem cells in the treatment of heart and vascular diseases (Chen et al., 2004; Hu et al., 2008; Nesselmann et al., 2008; Watt et al., 2013), the current cell therapy is not efficient due to the low survival and differentiation potential of stem cells after transplantation. Further investigations are needed to optimize cell therapy efficacy (Murry et al., 2004; Huang et al., 2010; van der Bogt et al., 2012; Der Sarkissian et al., 2017; Muller et al., 2018). Different strategies can be used to improve stem cell-based therapies, including pre-treatment of stem cells with cytokines and growth factors, tissue engineering, and genetic manipulation of the cells (Muller et al., 2018).
Apelin and its receptor express in different parts of the cardiovascular system, including the adult cardiac endothelium, atria, and endothelial cells (Szokodi et al., 2002; Kleinz and Davenport, 2004; Kleinz et al., 2005). The role of this peptide in heart development has been demonstrated (Zeng et al., 2007; Kang et al., 2013). Disturbance in apelin signaling leads to a defective vascular patterning (Cox et al., 2006) and an abnormal heart and vessel formation (Kang et al., 2013). In addition to the apelin role in cardiovascular development, it can affect cardiac function through different mechanisms. For instance, it exerts positive inotropic effects, improves cardiac function, and has antioxidant activity. Apelin protects cardiomyocytes against oxidative stress in ischemia/reperfusion injury and reduces cellular apoptosis (Szokodi et al., 2002; Berry et al., 2004; Atluri et al., 2007; Zeng et al., 2009; Azizi et al., 2013). It is involved in different signal transduction pathways regulating angiogenesis in hypoxic conditions (Novakova et al., 2016). Apelin also exerts protective effects against CVDs associated with obesity and diabetes. It regulates glucose and lipid metabolism in mice fed a high-fat diet (Ceylan-Isik et al., 2013). It protects cardiomyocytes against hyperglycemia-induced injury via its effect on cardiac gap junctions (Li et al., 2018), upregulates anti-aging proteins such as Sirtuins (Zeng et al., 2014; Grabowska et al., 2017), and eliminates oxidative stress (Foussal et al., 2010). Moreover, apelin treatment enhances the protective effects of stem cells on heart function (Li et al., 2013).
-
Apelin induces the cardiac differentiation of stem cells: One of the cardioprotective effects of MSCs is their ability to differentiate into cardiac cell lineages (Kajstura et al., 2005). Apelin may exert its protective role in the cardiovascular system by increasing the cardiac differentiation potential of stem/progenitor cells. Apelin-13 in combination with mesodermal growth factors increases cardiac differentiation of human and mouse ESCs (Wang et al., 2012). The silencing expression of apelin reduces the cardiac differentiation potential of WJ-MSCs mediated through the ERK pathway (Wang et al., 2016). This result is consistent with another report indicating the role of apelin in the cardiomyogenic differentiation of ESCs. D’Aniello et al. (2009, 2013) found that apelin is involved in cardiac differentiation of ESCs via the MAPK pathway. The expression of the APJ receptor regulates the differentiation process in ESCs, and its downregulation impairs cardiac differentiation of stem cells.
-
Apelin increases the paracrine effects of stem cells: Some scientists suggest that apelin-treated stem/progenitor cells participate in cardiovascular regeneration through angiogenesis and their paracrine effects but not via differentiation. Apelin increases the expression of factors associated with stem cell recruitment (such as SDF-1) in BMCs. Apelin-overexpressing BMCs increases the therapeutic effect of BMCs for myocardial infarction. They enhance the number of progenitor cells in the heart of post-MI mice more efficiently than BMCs (Li et al., 2013). Consistent with this report, Chung et al. (2016) demonstrated that apelin-13 could mobilize progenitor cells and improve the cardiac function after an acute myocardial injury. Moreover, after stem cell transplantation, increase in apelin expression in the heart or in the plasma of patients suffering from heart failure is associated with tissue repair. These findings show stem cells may participate in cardiac repair through a paracrine mechanism (Gao et al., 2009, 2010).
-
Apelin increases the angiogenic potential of stem cells: There is a relationship between EPC proliferation and angiogenesis. Hypoxia increases the EPCs proliferation in an apelin-dependent pathway. Overexpression of apelin in EPCs raises their angiogenic potential. These findings show the role of apelin in endothelial progenitor cell proliferation (Zhang et al., 2015, 2016). Apelin-overexpressing BMCs increases myocardial angiogenesis more efficiently than BMCs in post-MI mice (Li et al., 2013). Apelin increases the angiogenic potential of stem cells through the upregulation of factors related to angiogenesis, such as VEGF, in stem cells (Hou et al., 2017). Additionally, it cooperates with VEGF to form new blood vessels (Park et al., 2016).
-
Apelin reduces cellular death: A growing body of evidence suggests that apoptosis plays an important role in cell death after transplantation (Zhang et al., 2001; Geng, 2003; Zeng et al., 2012; Li et al., 2013). Therefore, the strategies that reduce apoptosis of the cells can improve the efficacy of stem cells therapy. Apelin exerts a protective effect against cellular apoptosis mediated by different signaling pathways (Tang et al., 2007). It also inhibits apoptosis induced by ischemia/reperfusion in cardiomyocytes (Zeng et al., 2009).
Apelin-13 enhances the efficacy of cell therapy by increasing the BM-MSCs survival and inhibiting cell apoptosis (Zeng et al., 2012). Moreover, it can improve the therapeutic potential of BMCs for cardiac diseases. Li et al. (2013) showed that delivery of apelin-overexpressing BMCs into the myocardium reduced myocardial apoptosis.
PAD is one of the leading causes of cardiovascular morbidity (Criqui and Aboyans, 2015). Stem cell therapy can be effective in the treatment of PAD through the activation of angiogenesis and cytoprotective pathways related to paracrine effects of stem cells (Frangogiannis, 2018). Apelin exerts a synergetic effect with cell therapy and promotes the therapeutic potential of stem cells by increasing angiogenesis and regulating autophagy. Although apelin administration or stem cell therapy can increase the perfusion ratio in the PAD model, their combination improves the treatment of PAD more efficiently. In the myocardial ischemia phase, activation of cardiac autophagy can improve cardiomyocyte survival by providing the energy needed for myocardial functions. However, in the reperfusion period, excessive activation of autophagy can lead to autophagic cardiomyocyte death. During the hypoxia phase, induction of protective autophagy can enhance the viability of stem cells against hypoxia and apelin boosts this process via activation of AMPK signaling and thereby promotes stem cell survival. In contrast, sustained autophagy during H/R may enhance cell death. Apelin, by increasing the AKT/Bcl2 signaling pathway, reduces authophagic cell death and protects the cells against H/R injury (Ma et al., 2015; Liang et al., 2016).
Apelin and stem cells: effects on energy metabolism
-
The effect on glucose metabolism:
Apelin can regulate glucose metabolism through different mechanisms. It reduces the glucose levels by increasing glucose uptake in muscle (Dray et al., 2008), affects the secretion of insulin (Sorhede Winzell et al., 2005), and increases the pancreatic insulin content in diabetic mice (Chen et al., 2011). In addition, there is a strong relationship between plasma insulin and apelin levels. During fasting, there is a reduction in the plasma insulin levels associated with a decrease in apelin messenger RNA level of adipocytes. Both levels are increased by refeeding. Plasma insulin regulates the expression of apelin in mouse adipocytes. It enhances the expression of apelin in adipocytes through the PI3K and PKC signaling pathways (Boucher et al., 2005). Moreover, pyr-Apelin-13 administration leads to an improved insulin sensitivity (Gourdy et al., 2018).
Recent investigations have shown that stem cell therapy has beneficial effects on diabetes (Hashemian et al., 2015). The transplantation of Wharton's jelly-derived MSCs alleviates type 2 diabetes mellitus in the patients and leads to an improved beta cell function. It reduces HbA1c and fasting glucose levels (Liu et al., 2014). Tsai et al. (2012) also demonstrated that UC-MSCs could differentiate into insulin-producing cells. Transplantation of differentiated cells reduced the level of glucose in diabetic rats.
It has been also demonstrated that apelin can enhance the therapeutic potential of stem cells for diabetes. Gao et al. (2018) reported that apelin-transduced stem cells exerted therapeutic effects on type 2 diabetic rats by promoting the proliferation of pancreatic β cells. Human WJ-MSCs overexpressing apelin reduce glucose levels and enhance the plasma levels of insulin and C-peptide. Additionally, apelin-overexpressing cells have the potential to improve insulin sensitivity in diabetic rats. There is a relationship between inflammation and insulin resistance. Suppression of interleukin 6 (IL-6) signaling leads to an improved insulin sensitivity (Shoelson et al., 2006; Schultz et al., 2010 ). Apelin-MSCs can decrease the levels of cytokines related to inflammation such as tumor necrosis factor-α and IL-6 in diabetic rats and thereby play a role in the improvement of insulin sensitivity (Gao et al., 2018).
-
The effect on lipid metabolism: Adipocytes express apelin and its receptor (Tatemoto et al., 2001; Boucher et al., 2005; Wei et al., 2005). The effects of apelin isoforms on lipid metabolism have also been revealed by a number of studies. Pyroglutamated apelin-13 reduces isoproterenol-stimulated lipolysis in adipocytes (Yue et al., 2011). Apelin-13 affects fatty acid oxidation and mitochondrial biogenesis in muscle (Attane et al., 2012).
The effect of apelin on adipogenic differentiation of stem cells has been demonstrated. Than et al. showed that pyr-apelin-13 suppressed adipogenesis of MSCs derived from adipose tissue. Apelin downregulates the expression of late makers of adipogenesis (such as aP2) in the differentiated cells. Also, apelin via MAPK/ERK-dependent pathways prevents adipogenesis of preadipocytes. This shows an inhibitory effect of apelin on adipogenesis (Than et al., 2012). Apelin is expressed in both white and brown adipose tissues (WAT, BAT) (Butruille et al., 2013), however, its role in brown adipogenesis is different than that in white adipogenesis. Indeed, apelin not only reduces the weight of white adipose tissue and hampers terminal differentiation of white adipocytes (Higuchi et al., 2007; Than et al., 2015) but also induces brown adipogenesis. Apelin administration increases differentiation of preadipocytes into brown adipocytes. It upregulates the markers related to thermogenesis and brown adipocyte in preadipocytes and enhances the numbers of differentiated brown adipocytes. It exerts these effects through the PI3K/Akt and AMPK signaling pathways (Than et al., 2015). Apelin also has a pivotal effect on the adipogenic differentiation of MSCs. Human BM-MSCs differentiate into white adipocytes with a low UCP1 expression. However, the administration of pyr-apelin13 leads to an increase in UCP1 expression in adipocytes derived from BM-MSCs (Than et al., 2015). These findings show that apelin could be considered as an activator to increase adipose browning and apelin-treated MSCs may play a crucial role in the treatment of metabolic-associated diseases.
Conclusion
This review summarizes the effects of the apelin/APJ system on the therapeutic potential of stem cells and their properties (Table 1). The mentioned studies show the beneficial effects of apelin on stem cell properties (Figure 1). Apelin administration affects stem cell survival and their differentiation potential through various signaling pathways (Figure 2). It improves the efficacy of stem cell therapy for CVDs. In addition, apelin exerts regulatory effects on energy metabolism via its effect on glucose and lipid metabolism. Although most of the reports have revealed the beneficial effects of the apelin signaling pathway on stem cells, further studies are needed to use apelin-treated stem cells in the treatment of different diseases. Among the different types of stem cells, most investigations have been performed on MSCs. The role of apelin signaling in other stem cell types has not been explored. The levels of the apelin isoforms in various stem cells remain to be understood. Apelin isoforms can activate different signaling pathways in stem cells. The effects of this activation on the fate of the cells and therapeutic potential of stem cells after transplantation have not been completely defined. More studies are needed to determine the advantages and disadvantages of apelin administration on stem cell therapy.
| References | Models | Intervention | Effects |
|---|---|---|---|
| Hou et al. (2017) | Mice BM-MSC culture in hypoxia | Stimulated with apelin-13 | Increase cell survival and proliferation under hypoxic condition. |
| Decrease cell apoptosis. | |||
| Increase angiogenesis, upregulation of VEGF. | |||
| Liang et al. (2016) | Mouse PAD model | Mice AD-MSCs transplantation with/without apelin-13 administration. | Improvement in hind limb blood perfusion in MSCs + apelin group. |
| Increase in angiogenesis. | |||
| Increase in cell survival. | |||
| Mice AD-MSCs culture | H/R injury in vitro + apelin-13 treatment | Improve cell survival via regulation of autophagy. | |
| Zhang et al. (2015) | EPC cell culture | Hypoxia treatment of EPCs | Apelin is involved in hypoxia-induced EPC proliferation via PI3K/AKT pathway |
| EPCs transfected with apelin plasmid/siRNA | |||
| Park et al. (2016) | Ischemic hind limb mouse model | Intramuscularly injection of human MSCs transfected with pDNA(VEGF)-coated apelin-17 loaded PLGA NPs | Blood flow recovery after injection of transfected cells. |
| Induce differentiation of MSCs into ECs. | |||
| Express EC-associated markers in transfected cells. | |||
| Accelerated neovascularization. | |||
| Li et al. (2014) | Rat BM-MSC culture | Incubation with apelin-13 | Increase of BM-MSC proliferation via AKT/GSK3b/cyclin D1. |
| Li et al. (2015) | Mice BM-MSC culture | Cultured under hypoxic conditions | Downregulation of APJ in BMCs reduces hypoxia-induced cell proliferation. |
| MSCs transfected with siRNA-APJ | |||
| Li et al. (2013) | MI mice model | Intramyocardial injection of BMC | Apelin-BMCs increase angiogenesis in heart of post-MI-mice. |
| Overexpressing apelin after MI | Apelin-BMCs reduce cardiac fibrosis and apoptosis. | ||
| Apelin-BMCs decrease ROS formation. | |||
| Improve heart functions | |||
| Zeng et al. (2012) | Rat BM-MSC culture | Treatment with apelin-13 | Improve cell survival. |
| Suppress cytochrome c release, reduce ROS formation. | |||
| Reduce cell apoptosis via ERK and AKT. | |||
| D′Aniello et al. (2009) | ESC lines | Cardiac differentiation | Upregulation of apelin and APJ during cardiac differentiation. |
| Apelin-13 treatment | Apelin enhances cardiomyogenesis via ERK pathway. | ||
| Wang et al. (2012) | Mouse/human ESC line | Cardiac differentiation | Apelin-13 exerts synergistic effects with mesodermal differentiation factors on cardiac differentiation of ESCs. |
| Apelin-13 treatment combined with mesodermal differentiation Factors | |||
| Boucher et al. (2005) | Mouse preadipose cell line (3T3F442A) | Adipocyte differentiation | The level of apelin mRNAs in preadipocytes increases during adipocyte differentiation. |
| Than et al. (2015) | Primary preadipocyte Culture | Adipocyte differentiation with | Apelin signaling enhances brown adipocyte differentiation via PI3K/Akt and AMPK. |
| human BM-MSCs culture | Pyr-apelin13 treatment | It upregulates thermogenesis marker, brown adipocyte-specific markers, and late marker of differentiated adipocyte in preadipocytes during differentiation. | |
| siRNA Silencing: gene silencing of apelin or APJ receptor in brown preadipocytes | Apelin increases UCP-1 expression in adipocytes derived from BM-MSCs. | ||
| Knocked down of apelin and APJ in brown preadipocytes by | |||
| siRNAs reduces brown adipocyte differentiation. | |||
| Chung et al. (2016) | Murine myocardial injury model | Apelin-13 administration intra-peritoneal injection of stem cells | Increase endothelial progenitor cells (EPCs) in circulation. |
| Decrease ischemic myocardium apoptosis. | |||
| Improve cardiac function. | |||
| Gao et al. (2010) | Rat myocardial Infarction models | Cardiomyogenic cell differentiation, | Cardiomyocytes differentiated from BM-MSCs express apelin. |
| Rat BM-MSCs culture | BM-MSC Injection | BM-MSCs transplantation improves cardiac function via paracrine effects. | |
| Gao et al. (2018) | Rat T2D model | Tail vein injection of human WJ-MSCs transduced with apelin-expressing | Improved survival in T2D rats, reduce glucose levels. |
| Lentiviral particles (WJ-MSCs-apelin) | Increase insulin sensitivity, increase in B cell numbers. | ||
| Reduce plasma inflammatory cytokines. |
- AD-MSCs, adipose derived mesenchymal stem cells; AMPK, AMP-activated protein kinase; APJ, apelin receptor, putative receptor protein related to the angiotensin receptor (AT1); BMCs, bone marrow cells; BM-MSCs, bone marrow-derived mesenchymal stem cells; EC, endothelial cells; EPCs, endothelial progenitor cells; ERK, extracellular signal-regulated kinases; ESC, embryonic stem cells; GSK3β, glycogen synthase kinase 3β; H/R injury, hypoxia/reoxygenation injury; MI, myocardial infarction; mRNA, messenger RNA; PAD, peripheral artery disease; pDNA-coated apelin-17 loaded PLGA NPs, pDNA-coated apelin-loaded poly-(DL)-lactic-coglycolic acid nanoparticles; PI3K/AKT, phosphoinositide 3 kinase/protein kinase B; ROS, reactive oxygen species; siRNA, small interfering RNA; T2D, type 2 diabetes; UCP-1, uncoupling protein 1; VEGF, vascular endothelial growth factor; WJ-MSCs, Wharton's jelly-derived mesenchymal stem cells.
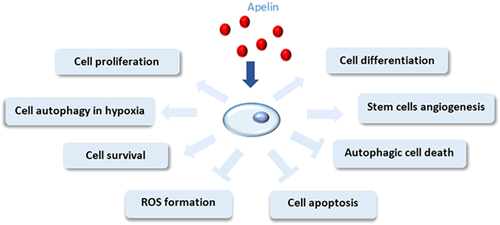
Apelin affects stem cell properties. Apelin increases the differentiation and proliferation potential of stem cells. It reduces reactive oxygen species (ROS) generation and suppresses cell apoptosis and autophagic cell death in stem cells, which improves stem cell survival.

Signaling pathways activated by apelin in stem cells. Apelin activates APJ (apelin receptor, putative receptor protein related to the angiotensin receptor (AT1)) receptors and stimulates or inhibits various signal transduction pathways. It induces activation of ERK/p70 S6K and thereby promotes cardiac differentiation of stem cells. It upregulates pro-survival and pro-angiogenic factors in stem cells, which increase the cell proliferation and survival. Apelin enhances cyclin D1 expression, thus increasing stem cell proliferation. Through activation of AKT/Bcl2, apelin inhibits autophagy-induced cell death in stem cells and promotes cell survival. It is also involved in brown adipogenesis via protein kinase B (AKT) and AMP-activated protein kinase (AMPK) pathways. Formation of reactive oxygen species (ROS) in stem cells decreases by apelin administration, and cell apoptosis is suppressed due to the apelin effect.
Conflict of interest
The authors declare that there are no conflict of interests.




