The role of vasodilator-stimulated phosphoprotein in podocyte functioning
Abstract
Vasodilator-stimulated phosphoprotein (VASP) is a 39-kDa protein belonging to the Ena/VASP protein family, which is involved in adhesion, migration, cell–cell interaction, and regulation of pathways connected with actin cytoskeleton remodeling. VASP is phosphorylated at Tyr39, Ser157, Ser239, Thr278, and Ser322 mainly by tyrosine kinase Abl, cAMP-dependent protein kinase, protein kinase G, AMP-activated protein kinase, and protein kinase D1, respectively. VASP phosphorylation, as a regulator of actin dynamics, may lead to impaired reorganization of the podocyte actin cytoskeleton not only by indirect interaction of VASP with actin but also by regulation of other signaling pathways. A few studies have shown that VASP participates in the development of renal diseases and mediates podocyte movement through its interaction with proteins of the slit diaphragm. VASP phosphorylation may cause reduced actin filament assembly in podocytes and mediate disturbances in regulation of filtration barrier permeability as a consequence of podocyte foot process effacement. In this paper, we describe the role of VASP in podocyte function, mainly in the context of actin dynamics and glomerular filtration barrier permeability. In addition, we discuss the involvement of VASP and its phosphorylated forms in the development of kidney diseases.
Abbreviations
-
- Abl
-
- tyrosine kinase Abl
-
- ActA
-
- actin assembly-inducing protein
-
- AMPK
-
- AMP-activated protein kinase
-
- CP
-
- capping protein
-
- EVH1
-
- Ena/VASP homology domain 1
-
- EVH2
-
- Ena/VASP homology domain 2
-
- Evl
-
- Ena/VASP-like protein
-
- FSGS
-
- focal segmental glomerulosclerosis
-
- GBM
-
- glomerular basement membrane
-
- PAR1
-
- protease-activated receptor-1
-
- PKA
-
- cAMP-dependent protein kinase
-
- PKD1
-
- protein kinase D1
-
- PKG
-
- protein kinase G
-
- PP
-
- protein phosphatases
-
- PRR
-
- proline-rich region
-
- SD
-
- slit diaphragm
-
- SOC
-
- store-operated Ca2+ channel
-
- TRPC6
-
- transient receptor potential canonical channel 6
-
- VASP
-
- vasodilator-stimulated phosphoprotein
-
- wt
-
- wild type
Introduction
Kidneys are among the most important excretory organs and responsible for eliminating waste products from the blood (Kokot, 1975). This function is possible thanks to the co-operation of many specialized cells that form the glomerulus, the filtering unit of the kidney. The glomerulus is a network of capillaries supported by intraglomerular mesangial cells, all enclosed in the Bowman's capsule (Pollak et al., 2014). The glomerular filtration barrier consists of the fenestrated endothelial cells, glomerular basement membrane (GBM), and podocytes (Arif and Nihalani, 2013). Podocytes are highly specialized cells consisting of a cell body, major foot processes, and interdigitated foot processes that completely cover glomerular capillaries and form filtration slits (Reiser and Altintas, 2016). The most important structure of the filtration barrier is the slit diaphragm (SD) that extends across the filtration slit and connects neighboring podocyte foot processes (Reiser and Altintas, 2016). The SD is a modified adherent junction that forms a signaling platform to regulate podocyte function and morphology (Huber and Benzing, 2005). The SD is composed of many proteins, including nephrin, podocin, TRPC6, and actin. Mutations in these proteins have been tied to diseases associated with foot process effacement and proteinuria (Fukasawa et al., 2009; Moller et al., 2009).
Foot process effacement is the retraction of foot processes and results from podocyte injury that alters actin cytoskeleton structure (Kriz and Lemley, 1999). The effacement involves the active rearrangement of actin filaments, regulated by proteins associated with the podocyte actin cytoskeleton. The precise control of actin organization is essential for maintaining the proper function of the cytoskeleton and its specialized structures in vivo. Assembling/disassembling of actin filaments and their spatial organization are coordinated by numerous actin-binding proteins (Quinlan, 2004; Disanza et al., 2005). Among these are vasodilator-stimulated phosphoprotein (VASP), a 39-kDa protein of the Ena/VASP family of actin-regulatory proteins, which share homology with mammalian Mena (the homologue of Drosophila Ena) and Evl (Ena/VASP-like protein) (Laurent et al., 1999).
VASP was first characterized in endothelial cells and platelets (Halbrügge and Walter, 1989). In addition, VASP is highly expressed in spleen, stomach, intestine, lung, and kidney, but its exact role in these tissues is unknown (Gambaryan et al., 2001).
VASP contains three domains: N-terminal Ena/VASP homology domain 1 (EVH1), a central proline-rich region (PRR), and C-terminal Ena/VASP homology domain 2 (EVH2) (Chereau and Dominguez, 2006; Blume et al., 2007). EVH1 is responsible for binding VASP to the proline-rich motifs of proteins associated with the cytoskeleton, such as vinculin, zyxin, lamellipodin, and actin assembly-inducing protein (ActA), which is the protein of the intracellular bacterial pathogen Listeria monocytogenes (Callebaut et al., 1998; Hansen and Mullins, 2015; Acevedo et al., 2017). The PRR contains binding sites for profilin and proteins containing WW (named for a conserved Trp-Trp motif) and Src homology 3 (SH3) domains (Holt et al., 1998; Krause et al., 2003). The EVH2 domain contains a G-actin–binding site, an F-actin–binding site, and a coiled-coil motif required for oligomerization of Ena/VASP proteins (Bachmann et al., 1999; Harbeck et al., 2000).
VASP plays a key role as a molecular adaptor-related to dynamic membrane activity and is involved in cell adhesion and migration (Wu and Gunst, 2015). Moreover, it acts as a regulator of proteins associated with remodeling of the actin cytoskeleton (Hütte lmaier et al., 1999; Harbeck et al., 2000; Walders-Harbeck et al., 2002; Galler et al., 2006; Benz et al., 2009). In this paper, we describe the role of VASP in podocyte functioning, including how changes in VASP phosphorylation can affect the development of glomerular diseases through the remodeling of the actin cytoskeleton in podocytes.
Actin dynamics and its role in podocyte functioning
The actin cytoskeleton is a complex organization of actin filaments (F-actin) with their accessory and regulatory proteins, which is the main source of force-generating machinery in the cell (Blanchoin et al., 2014; Svitkina, 2018). The cytoskeleton plays a key role in the production of contractile forces through the interaction of actin filaments with myosin II, and in cell division, facilitation of harmonized cell movement driven by filopodia and lamellipodia, and coordination of endocytosis and phagocytosis (Arber et al., 1998; Mooren et al., 2012). Moreover, the actin cytoskeleton is responsible for maintaining cell shape and the mechanical features of the cell surface, driving the intracellular movement of membrane organelles and providing cell–cell adhesions and interaction with the extracellular matrix (Galler et al., 2006; Trichet et al., 2008; Svitkina, 2018).
The cytoskeleton also determines podocyte shape and function. The cell body consists mainly of microtubules and vimentin intermediate filaments, whereas foot processes contain thick bundles of F-actin surrounded by branched actin filaments that run cortically and contiguously connect to neighboring processes (Noris and Remuzzi, 2012; Neal, 2015). This composition is tied to function. Podocyte foot processes link these cells and attach them through focal adhesions to the GBM, whereas beneath the podocyte cell body or its processes there is a space called the subpodocyte space (Neal et al., 2005; Neal et al., 2007; Kriz et al., 2013). Reorganization of the actin cytoskeleton is considered to be the contractile force that mediates active modulation of filtration barrier permeability through alterations in foot process morphology. Podocyte injury or stress leads to the formation of a dense network of dislocated filaments and is associated with foot process effacement characterized by the loss of the interdigitating pattern of adjacent foot processes and of the SD (Noris and Remuzzi, 2012; Kriz et al., 2013). Foot process effacement is considered to be the podocyte's compensatory and adaptive mechanism to protect cells against detachment. The loss of mechanisms responsible for maintaining attachment leads to the detachment of a viable podocyte from the GBM and it is excreted in the urine (Vogelmann et al., 2003).
The SD is connected to the podocyte actin cytoskeleton through multiple adaptor proteins, including α-actinin, CD2AP, synaptopodin, and zonula occludens-1 (Faul et al., 2007). Proteins that are essential for the proper functioning of SD include nephrin and podocin. Nephrin is a transmembrane protein with a short intracellular portion and an extracellular segment with IgG- and fibronectin-type III–like motifs. This protein interacts with the podocyte actin cytoskeleton and serves as a scaffold for the SD (Patrakka and Tryggvason, 2007). Nephrin phosphorylation leads to the recruitment of Nck protein to the intracellular domain and promotion of localized actin polymerization (Lehtonen, 2008). At the SD, nephrin interacts with podocin, which is an integral membrane protein and forms highly organized oligomeric structures associated with lipid rafts (Shono et al., 2007).
There is a strong relationship between nephrin, podocin and the podocyte actin cytoskeleton, where both nephrin and podocin are involved in foot process formation by affecting actin filaments (Saleem et al., 2002). Loss of nephrin or podocin leads to proteinuria, a manifestation of foot process effacement (Kestilä et al., 1998; George and Holzman, 2012). Evidence shows that nephrin and podocin are associated with the actin cytoskeleton and affect its organization, but the exact mechanism is unknown. However, an earlier report (Harris et al., 2013) has indicated that podocin interacts with VASP, which may influence actin dynamics and actin cytoskeleton reorganization.
The role of VASP in regulating actin dynamics
Actin filament consists of globular actin that undergoes continuous assembly and disassembly during the mechanism of treadmilling (Lee and Dominguez, 2010). In this process, the filamentous actin is elongated by reversible addition of actin monomers (G-actin) to the fast polymerizing end (barbed end) faster than an addition to the slow polymerizing end (pointed end). The actin monomers with adenosine triphosphate bind to the barbed end of the existing actin filament, while monomers with adenosine diphosphate are detached from the pointed end (Cooper and Sunderland, 2000; Schüler et al., 2006; Interliggi et al., 2007).
One of the functions attributed to VASP is the anti-capping activity that allows for elongation of the actin filament, even if capping proteins (CPs) are present. This mechanism is based on associating Ena/VASP proteins with elongating F-actin at/near their barbed end in a way that inhibits CP action (Bear and Gertler, 2009). An earlier study (Breitsprecher et al., 2008) confirmed the anti-capping function of VASP, which can support continuous F-actin elongation even in the presence of CP. Moreover, (Applewhite et al., 2007) suggested a hypothesis that filopodia formation is initiated by Ena/VASP proteins. In the beginning, the Ena/VASP proteins function to cross-link or aggregate barbed ends of actin through tetramerization and at the same time Ena/VASP interacts with F-actin (Applewhite et al., 2007). This is consistent with the fact that Ena/VASP is able to bundle F-actin (Bachmann et al., 1999; Hüttelmaier et al., 1999; Laurent et al., 1999). In the next step, Ena/VASP is temporarily recruited to the membrane-bound ligands located on the leading edge to stabilize the full-length F-actin at filopodial tips (Applewhite et al., 2007). To allow for continuous polymerization by the insertion of G-actin, the interaction between GAB motif of Ena/VASP and F-actin barbed ends is transient in filopodia (Applewhite et al., 2007).
Ena/VASP proteins also can nucleate F-actin in vitro. The Ena/VASP-dependent nucleation of F-actin was confirmed through using pyrene-actin polymerization assay (Hüttelmaier et al., 1999; Skoble et al., 2001). However, in vivo, it is unlikely that VASP is a physiological nucleator of actin because this process is extremely dependent on salt content and usually takes place at subphysiological salt concentrations (Bear and Gertler, 2009).
Furthermore, numerous studies have shown that Ena/VASP proteins restrict F-actin branching by Arp2/3 complex (Bear et al., 2002; Samarin et al., 2003; Sechi and Wehland, 2004); however, the Ena/VASP direct effect on branching is unknown (Skoble et al., 2001; Bear and Gertler, 2009). It is hypothesized that Ena/VASP proteins compete with the Arp2/3 complex for actin monomers (essential cofactors for branching) (Bear and Gertler, 2009). Another likely hypothesis for anti-branching ability of Ena/VASP is that increased CP activity augments branching as a result of locally increasing levels of actin monomers (Akin and Mullins, 2008). It is predicted that Ena/VASP with its anti-capping function may decrease branching by the diminished capping of barbed ends, which could lead to increased consumption of G-actin by its addition to barbed ends (Bear and Gertler, 2009). Unfortunately, any of these mechanisms have not been investigated in podocytes, so to date, this issue remains unresolved.
Regulation of VASP function
VASP phosphorylation depends on intracellular localization, the availability of phosphorylation sites in VASP for other proteins, accessibility of particular protein kinases and/or protein phosphatases (PP), and the individual activators and inhibitors of these phosphatases and kinases (Calzi et al., 2008). In addition, phosphorylation is a convenient mechanism of control that is fast and effective, especially with a protein that is constitutively present within cells.
VASP has five identified phosphorylation sites: Tyr39, Ser157, Ser239, Thr278, and Ser322 (Figure 1) (Döppler and Storz, 2013). The function of VASP is regulated mainly by serine/threonine kinases such as cAMP-dependent protein kinase (PKA), protein kinase G (PKG), AMP-activated protein kinase (AMPK), and protein kinase D1 (PKD1). A few years ago tyrosine kinase Abl (Abl) joined this group (Thomson et al., 2011; Maruoka et al., 2012; Döppler and Storz, 2013).
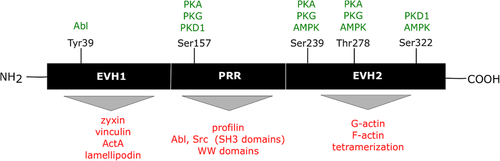
Organization of human vasodilator-stimulated phosphoprotein (VASP), localization of phosphorylation sites, and overview of proteins interacting with VASP (Döppler and Storz, 2013; modified).
VASP is phosphorylated by PKA at Ser157 with faster kinetics than Ser239 and Thr278. Phosphorylation of VASP at Ser157 leads to changes in VASP localization within the cell without affecting actin filament formation or the G-actin/F-actin ratio and inhibits VASP interaction with the SH3 domains of Abl kinase and nSrc (Harbeck et al., 2000; Pula and Krause, 2008; Benz et al., 2009). Phosphorylation of VASP at this site (Ser157) is probably necessary for targeting VASP to its potential functional sites within the cell (Benz et al., 2009). VASP is also phosphorylated by PKD1 at Ser157 (Döppler et al., 2013). Protein kinase C and Rho-associated protein kinase 1 are upstream of PKD1 in RhoA-signaling pathways, and it is probable that PKD1 contributes to protein kinase C–dependent VASP phosphorylation (Döppler and Storz, 2013).
As noted, VASP is also a substrate for PKG, which in the first instance phosphorylates Ser239 and then Ser157 (Smolenski et al., 1998; Zhang et al., 2010). Studies by Routray et al. (2011) revealed that phosphorylation of Ser157, Ser239, and Thr278 through PKG disturbs Rac1-dependent focal adhesion assembly in hepatic stellate cells.
The next VASP phosphorylation site is Thr278. This amino acid is phosphorylated by both PKA and PKG, but only following phosphorylation of VASP at Ser157 and Ser239. A recent study has shown that AMPK prefers Thr278, and this reaction causes reduced F-actin assembly and consequently impaired stress fiber formation and altered cell morphology (Blume et al., 2007). Another newly characterized phosphorylation site preferred by AMPK and PKD1 is Ser322. Phosphorylation at this site increases F-actin accumulation and promotes filopodia formation (Thomson et al., 2011; Döppler et al., 2013).
Abl is another kinase that phosphorylates VASP at Tyr39 and limits VASP localization in focal adhesions (Maruoka et al., 2012). Moreover, Abl is one of the activators of PKD1 during oxidative stress. It is possible that Abl regulates VASP function by its phosphorylation at Tyr39 in a PKD1-dependent manner, an issue worth investigating (Döppler and Storz, 2013).
Little is known about the mechanisms of VASP dephosphorylation. This process has been studied only in platelets, where inactivation of VASP is implemented by PPs: PP2A, PP2B, and PP2C in vitro (Abel et al., 1995). PP2A completely dephosphorylates VASP, whereas PP2B and PP2C dephosphorylate VASP at Ser239 and Thr278 sooner than at Ser157 (Abel et al., 1995).
Phosphorylation and dephosphorylation are important regulatory mechanisms of VASP function that affect the actin cytoskeleton. Understanding these patterns allows for defining the pathological mechanisms responsible for disturbances in actin organization and disease development.
The role of VASP in podocytes
Experimental evidence shows that VASP is expressed in the kidney, including mesangial cells, myofibroblasts in the cortex, interstitial cells, and podocytes (Gambaryan et al., 2001; Hohenstein et al., 2005). Unfortunately, the exact role of VASP and its phosphorylation in the whole kidney and particular cells of a healthy kidney, especially in podocytes, is not known. Only a few experiments have indicated the role of VASP in podocyte function.
The role of VASP in vivo has been studied in induced passive crescentic nephrotoxic nephritis. Kidneys of healthy VASP-deficient and wild-type (wt) mice were compared with kidneys of VASP-deficient and wt mice with the disease, respectively (Hohenstein et al., 2005). In healthy mice, VASP was found in blood vessel walls and in interstitial and glomerular mesangial cells. During nephritis, VASP expression markedly increased in podocytes. Progression of nephrotoxic nephritis in wt mice after disease induction was characterized by glomerulosclerosis, glomerular injury, and loss of podocytes. Progression of chronic renal disease observed in wt mice could also be observed but to a lesser degree in VASP-deficient mice, in which kidney's structures like glomeruli and podocytes were significantly protected against the nephrotoxic nephritis for a long time (Hohenstein et al., 2005). The active rearrangement of the actin cytoskeleton is involved in the process of podocyte detachment, whereas VASP is a regulator of actin dynamics (Faul, 2014). Unfortunately, there are no data about sites of VASP phosphorylation in podocytes with nephrotoxic nephritis and its influence on disease development. It is possible that the loss of podocytes during nephrotoxic nephritis is the result of VASP phosphorylation, which may impair the formation of F-actin and may contribute to the effacement of foot processes. The results of other studies have shown that VASP phosphorylation at Ser239 (pVASP Ser239) and Thr278 (pVASP Thr278) leads to alteration in actin filament accumulation (Harbeck et al., 2000; Benz et al., 2009). Studies by Blume et al. (2007) also indicated that pVASP Thr278 negatively regulates F-actin assembly and reduces the formation of stress fibers. Diminished formation of stress fibers that support podocyte foot processes leads to foot process effacement and may be the reason for proteinuria (Saleem et al., 2002).
The protease-activated receptor-1 (PAR1), like other PARs (PAR2, PAR3, PAR4), is a transmembrane receptor that belongs to the family of G protein-coupled receptors and is activated in response to proteolytic cleavage induced by agonist proteinases (Hirano and Kawabata, 2008). Thrombin is a serine protease that cleaves the N-terminal extracellular region of PAR1 and this cleaved part interacts with the PAR1 to induce transmembrane signaling (Hirano and Kawabata, 2008). Human podocytes express PAR2, PAR3, and PAR4, whereas rat podocytes express all PARs (Sharma et al., 2017). Sharma et al. (2017) demonstrated that the generation of thrombin in plasma increases during nephrotic syndrome and inhibition of this enzyme leads to a decrease in proteinuria in experimental nephrotic syndrome. Moreover, thrombin mediates reorganization of the actin cytoskeleton and induces injury to rat podocytes in a PAR1- and PAR4-dependent manner (Sharma et al., 2017). Recent studies have demonstrated that PAR1 expression of messenger RNA in a mouse model of nephropathy and diabetic nephropathy is significantly elevated (Waasdorp et al., 2016; Guan et al., 2017). Furthermore, inhibition of PAR1 reduces albuminuria and podocyte injury in vivo in the mouse model of streptozotocin-induced diabetic nephropathy (Guan et al., 2017).
PARs may be involved in signaling to the actin cytoskeleton. A recent study has demonstrated that PAR1 signaling to VASP depends on the correct localization of podocin (Harris et al., 2013). They showed that in podocytes incubated with FSGS (focal segmental glomerulosclerosis) plasma containing proteases, VASP is phosphorylated at Ser157 and Ser239 in response to PAR1. In contrast to these data, in podocin-mutant podocytes, PAR1 does not phosphorylate VASP. Moreover, podocytes treated with nephrotic plasma are characterized by increased VASP phosphorylation and enhanced cell migration (Harris et al., 2013). In summary, activation of PAR1 promotes VASP phosphorylation through podocin and increases podocyte motility.
Similar results were reported in endothelial cells, in which VASP phosphorylation at Ser157 also increases their motility (Zhang et al., 2010). These results suggest that VASP phosphorylation is associated with the actin cytoskeleton and its reorganization. Furthermore, VASP-dependent actin reorganization can be regulated by podocin, which is an integral component of the SD. Disturbances in SD functioning may impair signal transduction to the actin cytoskeleton, and podocytes may not be able to properly regulate filtration barrier permeability in response to the signal.
Like podocin, FAT1 is a part of the SD and belongs to the cadherin superfamily (Yaoita et al., 2005). Studies by Moeller et al. (2004) have indicated that FAT1 binds to Ena/VASP proteins and promotes actin polymerization at the leading edge of kidney epithelial cells. The same mechanism may be present in podocytes and regulate podocyte actin dynamics.
Another important component of the SD is transient receptor potential canonical channel 6 (TRPC6), which is an important calcium channel in podocytes (Reiser et al., 2005; Winn et al., 2005). Data show that TRPC6 is associated with the podocyte actin cytoskeleton, which is reorganized after TRPC6 overexpression or insulin treatment (He et al., 2013; Rogacka et al., 2017). We showed that in insulin-treated podocytes, calcium influx is attenuated by inhibition of TRPC6 activity through activation of protein kinase G Iα (PKGIα) (Rogacka et al., 2017). Moreover, insulin plays an important role in glucose uptake, it regulates actin organization in podocytes and is involved in preserving the integrity of the glomerular filtration barrier (Welsh et al., 2010; Coward and Fornoni, 2015). Knocking down of insulin receptor in mice podocytes leads to the loss of their foot processes. Furthermore, these mice develop albuminuria concomitantly with changes typical for diabetic nephropathy, which shows the contribution of insulin signaling to the regulation of the glomerular permeability (Welsh et al., 2010).
In our papers, we demonstrated that insulin increases filtration barrier permeability through TRPC6-dependent activation of PKGIα signaling pathways in podocytes (Piwkowska et al., 2013; Piwkowska et al., 2015; Rogacka et al., 2017). Maybe VASP is involved in this process by phosphorylation of this protein by PKGIα. We noted that PKGIα is localized perinuclear and VASP seems to be less evenly distributed in control podocytes than in cells after insulin treatment. Moreover, the intensity of VASP signal is markedly higher after incubation of podocytes with insulin than in the control cells. Additionally, for the first time we showed that VASP significantly colocalizes with PKGIα after insulin treatment (from 29.72% ± 0.02% to 82.05% ± 0.15%, n = 3, P < 0.05, paired t student, Figure 2). These results may suggest that insulin regulates podocyte permeability through rearrangement of the actin cytoskeleton as a consequence of VASP phosphorylation in a PKGIα-dependent manner.
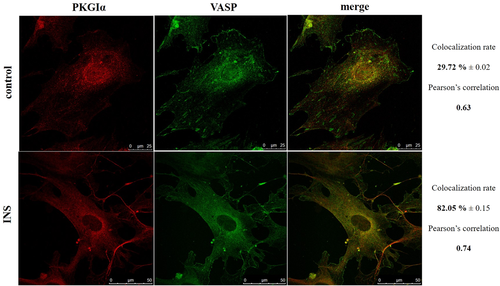
Subcellular distribution of protein kinase G Iα (PKGIα) and vasodilator-stimulated phosphoprotein (VASP) in cultured rat podocytes. The merged images show colocalization of PKGIα with VASP in differentiated rat podocytes. Cells seeded onto coverslips were incubated for 5 min in the absence or presence of insulin (INS, 300 nM). Podocytes were then immunostained with anti-PKGIα (1:15; Santa Cruz Biotechnology) and anti-VASP (1:30; Sigma Aldrich) antibodies. Quantitative analysis of protein colocalization was performed with LAS AF 3.3.0 software (n = 3, P < 0.05). The pixel intensities were quantified and the results are presented as Pearson's correlation coefficients and colocalization rates (%).
Studies conducted on glomerular mesangial cells indicated that TRPC4, a protein in the same family as TRPC6, is negatively regulated by activation of PKGIα. Of note, VASP is involved in this response (Wang et al., 2007). Phosphorylation of VASP at Ser239 by PKGIα leads to interaction between pVASP Ser239 and TRPC4. VASP does not interact with TRPC4 if it is not phosphorylated (Wang et al., 2007). These authors inferred that the association of pVASP Ser239 with TRPC4 is responsible for TRPC4 inhibition, probably by dissociation of pVASP Ser239-TRPC4 from the SOC (store-operated Ca2+ channel) complex (O’Neil, 2007). It is highly probable that the same mechanism connected to VASP-dependent regulation of the SOC complex may occur in podocytes and affect the regulation of filtration barrier permeability.
Additionally, VASP seems to be responsible for modulating signaling at the actin cytoskeleton (Walders-Harbeck et al., 2002; Galler et al., 2006; Chen et al., 2008). It regulates Rac and PAK activity, proteins that are associated with regulation of the actin cytoskeleton dynamic, and participates in F-actin assembly (García Arguinzonis et al., 2002; Galler et al., 2006). Previous studies demonstrated that Ena/VASP-deficient endothelial cells are not able to reorganize stress fibers under physiological shear stress and that G-actin incorporation at barbed ends of stress fibers is diminished (Furman et al., 2007). Moreover, Ena/VASP deficiency is correlated with increased endothelial permeability (Furman et al., 2007). Another study showed that VASP is involved in stabilization of endothelial barrier through cAMP-dependent activation of Rac1 (Schlegel et al., 2008; Schlegel and Waschke, 2009). In fibroblasts and mesangial cells, VASP elimination enhances spreading (García Arguinzonis et al., 2002; Galler et al., 2006). Moreover, it was demonstrated that activation of Rac1/PAK pathway, an important part in the regulation of cell spreading, is significantly increased in VASP-deficient fibroblasts (García Arguinzonis et al., 2002). Interestingly, mutation of VASP phosphorylation sites (Ser239Ala, Ser157Ala, and Thr278Ala), VASP overexpression or VASP knock out mediate the formation of stable and thick actin stress fibers in fibroblasts and mesangial cells (Galler et al., 2006). The Galler et al. (2006) suggested a hypothesis that increased Rac/PAK pathway activity in VASP-deficient cells leads to stabilization of stress fibers.
The Rho GTPase Rac1 and its effector PAK are also expressed in podocytes, and their activation usually promotes podocyte injury and detachment as the result of foot process effacement (Robins et al., 2017). Despite the unknown role of VASP in regulating the signaling pathways in podocytes, disturbances in VASP function may be involved in the regulation of rearrangement of the podocyte actin cytoskeleton through activation of the Rac1/PAK pathway.
Conclusions
Although the structure of VASP has been studied and described, the mechanism of action and the particular role of VASP in podocytes and kidney are poorly understood. VASP and its phosphorylation play an important role in regulating actin dynamics. A broad body of literature shows that VASP phosphorylation leads to disturbances in F-actin assembly and mediates regulation of other proteins associated with actin cytoskeleton rearrangement. In podocytes, earlier findings suggest that VASP phosphorylation contributes to increased motility and interacts with PKGIα after insulin stimulation. Understanding the role of VASP in cell signaling in podocytes may help with predicting the mechanisms responsible for the development of kidney diseases and identify VASP as a novel therapeutic target for treating these diseases.
Acknowledgment
This work was supported by the National Science Centre, Poland (grant number 2014/14/E/NZ4/00358).
Conflict of interest
The authors declare that there are no conflict of interests.




