Nanocomposite scaffold seeded with mesenchymal stem cells for bone repair
Abstract
The mechanical property of bone tissue scaffolds is one of the most important aspects in bone tissue engineering that has remained problematic. In our previous study, we fabricated a three-dimensional scaffold from nano-hydroxyapatite/gelatin (nHA/Gel) and investigated its efficiency in promoting bone regeneration both in vitro and in vivo. In the present study, the effect of adding silicon carbide (SiC) on the mechanical and biological behaviors of the nHA/Gel/SiC and bone regeneration in vivo were determined. nHA and SiC were synthesized and characterized by the X-ray diffraction pattern and transmission electron microscope image. Layer solvent casting, freeze drying, and lamination techniques were applied to prepare these scaffolds. Then, the biocompatibility and cell adhesion behavior of the synthesized nHA/Gel/SiC scaffolds were investigated. For in vivo studies, rats were categorized into three groups: blank defect, blank scaffold, and rat bone marrow mesenchymal stem cells (rBM-MSCs)/scaffold. After 1, 4, and 12 weeks post-injury, the rats were sacrificed and the calvaria were harvested. Sections with a thickness of 5 µm thickness were prepared and stained with hematoxylin–eosin and Masson's Trichrome, and immunohistochemistry was performed. Our results showed that SiC effectively increased the mechanical properties of the nHA/Gel/SiC scaffold. No significant differences were observed in biocompatibility, cell adhesion, and cytotoxicity of the nHA/Gel/SiC in comparison with the nHA/Gel nanocomposite. Based on histological and immunohistochemical studies, both osteogenesis and collagenization were significantly higher in the rBM-MSCs/scaffold group, quantitatively and qualitatively. The present study strongly suggests the potential of SiC as an alternative strategy to improve the mechanical and biological properties of bone tissue engineering scaffolds, and shows that the pre-seeded nHA/Gel/SiC scaffold with rBM-MSCs improves osteogenesis in the engineered bone implant.
Abbreviations
-
- CaP
-
- calcium phosphate
-
- DPPC
-
- dipalmitoylphosphatidylcholine
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- GA
-
- glutaraldehyde
-
- H&E
-
- hematoxylin and eosin
-
- LDH
-
- lactate dehydrogenase
-
- nHA/Gel
-
- nano-hydroxyapatite/gelatin
-
- rBM-MSCs
-
- mesenchymal stem cells
-
- SEM
-
- scanning electron microscope
-
- SiC
-
- silicon carbide
-
- TEM
-
- transmission electron microscope
-
- XRD
-
- X-ray diffraction
Introduction
One of the main problems in the orthopedic field is large bone defects. In critical bone lesions, the bone repairing process is faced with a great challenge, which does not result in complete regeneration in most cases (Mobini et al., 2013a; Danoux et al., 2014; Arakawa et al., 2017). Currently, there is a considerable demand for organ replacement as a result of the annual increase of the world's population. Tissue engineering is an exciting field that aims to repair damaged tissues and restore their functionality through application of various biomaterial scaffolds, cells, growth factors, and cytokines (Shin et al., 2003; Gholipourmalekabadi et al., 2015d). Despite remarkable progress in this context, some challenges still remain unsolved.
An optimal three-dimensional (3D) scaffold for use in bone tissue engineering should have sufficient porosity with inter-connective pores (at least 100 µm in diameter) to facilitate cell ingrowth, migration, proliferation, and differentiation. In addition, such a scaffold should have suitable mechanical properties to mimic natural bone characteristics (Hamlehkhan et al., 2012; Gholipourmalekabadi et al., 2015c; Kouhi et al., 2016; Diomede et al., 2018). Current bone substitutes available on the market are still not optimal, and many synthesized bone grafts have different limitations. Therefore, efforts are continuing to develop an ideal bone graft. Many materials and methods have been used for fabrication of various bone substitutes (Poursamar et al., 2009; Hamlehkhan et al., 2012; Johari et al., 2012; Samadikuchaksaraei et al., 2016; Gugliandolo et al., 2018). Calcium phosphate (CaP)-derived materials such as bioactive hydroxyapatite [HA, Ca10 (PO4)6(OH)2] are among the most widely used biomaterials. Although HA has some chemical and structural properties that make it very similar to natural bone, it should be combined with certain organic components to mimic both the organic and inorganic phases of bone. For example, composites of HA/Collagen (Liao et al., 2004), HA/Chitosan (Yamaguchi et al., 2001), HA/Collagen/Poly (lactic acid) (Liao et al., 2005), HA/Chitosan-Alginate (Jin et al., 2012), and HA/Gelatin (Johari et al., 2016a; Samadikuchaksaraei et al., 2016) have been frequently used for bone regeneration.
Silica-based materials such as silicon carbide (SiC) can facilitate cell migration and cellular activity through augmentation of extracellular deposition (Poursamar et al., 2009; Johari et al., 2016a). These biomaterials show excellent mechanical properties (Munoz et al., 2002; Singh and Salem, 2002) and increase the rate of bone formation and mineralization.
Utilization of bioactive materials in the structure of scaffolds is the other important aspect of bone tissue engineering. Mesenchymal stem cells (MSCs) present in many tissues could be easily isolated and cultured. These cells are able to differentiate into the bone-forming cells. Furthermore, their use on a large scale is not associated with the risk of provoking a host immune response (Salgado et al., 2004; Wang et al., 2006). MSCs exhibit more advantages than other cell sources that have been used in bone repair (Fisher and Reddi, 2003).
In a previous study (Azami et al., 2010b), a 3D nHA/Gel nanocomposite was fabricated and its characteristics in vitro and in vivo were determined. The aim of the present study was to reinforce the nHA/Gel scaffolds with SiC for bone tissue engineering applications. The effects of adding SiC on the mechanical properties, microstructure, and cellular response were determined. Furthermore, an in vivo study was carried out using rat bone marrow mesenchymal stem cells (rBM-MSCs) cultured on the developed scaffold, as an implant, to improve bone repair in a rat calvarial bone defect model.
Materials and methods
Preparation of nano-hydroxyapatite (nHA)
The nHA particles were synthesized, as described in our previous publication (Ramedani et al., 2014). Briefly, dipalmitoylphosphatidylcholine (DPPC) and cholesterol were dissolved in chloroform, and stirred for 30 min at 40°C. For preparation of the nHA particles, calcium nitrate tetrahydrate [Ca (NO3)2.4H2O] and diammonium hydrogen phosphate [(NH4)2HPO4] were used as the source of calcium and phosphate ions, respectively. An aqueous solution of calcium salt was prepared using deionized water and added to the dry film lipid and mixed at a temperature of 51°C for 2 h (the pH was adjusted at 4.5 using acetic acid). The prepared solution was then sonicated for 30 min to disperse the particles in the liquid media and form nano-sized structures. The aqueous solution of sodium phosphate prepared as deionized water drops was added to the solution of calcium and lipids. The milky suspension was placed inside an oven for 22 h at 120°C. At the end of the hydrothermal process, the suspension was centrifuged; the precipitate was washed with ethanol/deionized water, and dried at a temperature of 90°C. The dried precipitate was hand ground to prepare the nano-powder for the downstream experiments.
Preparation of nanocomposite scaffolds
The nHA/Gel scaffolds were fabricated according to a previously reported protocol (Ali Samadikuchaksaraei and Ghafouri, 2009) Briefly, a final weight composition of 60%-Gel and 40%-nHA + SiC was achieved by adding the appropriate amount of nHA powder to a 10% (wt/vol) solution of Gelatin (microbiology grade; Merck, Germany). The solution was cast in plastic Petri dishes after being completely homogenized by stirring. The solution was freezed at −20°C for 60 min and then freeze-dried to produce a porous structure through solvent sublimation. The nanocomposite layers were cut into the desired sizes and laminated by applying a gel solution of 10% wt/vol as a binding agent. The sample was then soaked in a 1% (wt/vol) glutaraldehyde (GA) solution for 24 h to cross-link the gel polymeric chains. Then, the nanocomposite was washed three times with deionized distilled water and dried using freeze drying. To prepare the SiC-containing nanocomposite scaffold, a similar method was applied. In this case, SiC particles were also added to the gel solution after nHA. The amount of SiC and nHA were adjusted versus the gel to finally obtain a weight composition of 30% nHA, 10%SiC, and 60% Gel. The next steps were carried out exactly the same as for the previously described scaffold lacking SiC.
Characterizations of nHA/Gel/SiC scaffold
X-ray diffraction (XRD) analysis
The resulting powder was analyzed by XRD with a Siemens-Brucker D5000 diffractometer. This instrument works with voltage and current settings of 40 kV and 40 mA, respectively, and uses Cu-Kα radiation (1.540600 Å). In order to qualitatively analyze, XRD diagrams were recorded in the interval 20 ≤ 2θ ≤ 80° at a scan speed of 2°/min, the step size being 0.02° and the step time 1 s.
Transmission electron microscope (TEM)
A TEM (Philips CM200 model) operating at an acceleration voltage of 200 kV was used to evaluate the nanoparticles. In addition, the particle size distributions were measured by importing the TEM images into ImageJ technical software (Developed by Wayne Rasband, NIH, USA).
Scanning electron microscope(SEM)–energy-dispersive X-ray
The microstructure of the scaffolds was observed by using an SEM. For this purpose, both nHA/Gel and nHA/Gel/SiC nanocomposite scaffolds were sputter coated with gold (Au) and viewed under SEM (AIS2100; Seron Technology, Uiwang-si, Gyeonggi-do, South Korea) at an acceleration voltage of 25Kv. The Pores’ diameter (average of ten pores) of the samples (n = 5) was measured by SEM software.
Porosity and density
The porosity and density of the scaffolds were defined by the following formulae as described in our previously published study (Gholipourmalekabadi et al., 2015c):
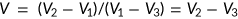 (1)
(1)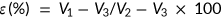 (2)
(2)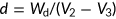 (3)
(3)Mechanical analysis
The mechanical behavior of the prepared dry scaffolds was studied by conducting a compressive test according to ASTM F 451-86. Toward this goal, scaffolds were cut to 6 × 12 mm. Dimensions were measured with an electric digital caliper. This test was performed using a Roel-Amstel mechanical instrument with a strain rate of 1 mm/min. Each test was repeated four times and the average amount and standard deviation (SD) of related parameters such as E (Young's modulus) and yield strength (σ yield) were calculated.
Cellular responses
The cellular response of both nHA/Gel and nHA/Gel/SiC nanocomposites was evaluated by culturing rBM-MSCs on the scaffolds. The short-term cell viability, cytotoxicity and cell adhesion property were studied by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), lactate dehydrogenase (LDH) assay and SEM micrographs, respectively.
rBM-MSCs isolation
rBM-MSCs were isolated from the rat bone marrow and expanded by using a protocol described in our previously published study (Gholipourmalekabadi et al., 2015d). In a sterile condition, both ends of the femur bone were excised and the bone marrow was flushed out into a cell culture flask of 25cc with a needle loaded with fetal albumin serum (10%), 100 ng/mL penicillin (Gibco, Carlsbad, CA), and 100 ng/ml streptomycin (Gibco). Then, the cell culture flask was incubated overnight at 37°C, 5% CO2 and 95% humidity. Once in every 2 days, the medium containing non-adherent cells was removed and replaced with fresh media. Cell passage was carried out once the cultured cells reached 80–90% confluency.
Characterization of rBM-MSCs
Flow cytometry was used to verify the characterization of the rBM-MSCs. Cells from the third passage were counted and 4 × 105 cells were incubated with monoclonal antibodies against surface antigens including CD90, CD105, CD34, and CD45.
To identify the differentiation ability of the rBM-MSCs in vitro, 1 × 105 cells/well of rBM-MSCs were plated in 12-well plates and cultured using osteogenesis and adipogenesis differentiation kits (Gibco). The medium was replaced every 72 h for 3 weeks. For osteogenic and adipogenic differentiation, the induced cells were analyzed with Alizarin red S (ScienCell, Carlsbad, CA) and oil red O (Sigma, St. Louis, MO), respectively (Xue et al., 2017; Huang et al., 2018).
Cell viability and cytotoxicity
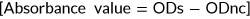 (4)
(4)LDH-specific activity of cell culture medium was measured using an LDH kit (Zist Shimi kit, Co No: 10–503 and 10–533-1, Iran), based on the p-nitrophenyl phosphate conversion to p-nitrophenol. The UV absorbance of nicotinamide adenine dinucleotide (NADH), as an index of NADH concentration, was quantitated on a Biotek EL800 absorbance plate reader at 490 nm. At the same time, the cells were ruptured via freeze thawing (three times) and the total LDH activity in the medium was measured. LDH data were normalized for 106 cells (Gholipourmalekabadi et al., 2015d).
Cell adhesion
Morphology of the rBM-MSCs cultured on the scaffold after 48 h was observed by SEM. The cell-nanocomposite complex was prepared for taking micrographs by SEM as described before (Mobini et al., 2013b; Gholipourmalekabadi et al., 2015a). The samples were fixed in 2.5% GA (Merck) for 2 h and then treated with 0.1% osmium tetroxide (Sigma-Aldrich) for 30 min at 25°C. After dehydrating in a graded concentration (30, 50, 70, and 100%) of acetone (Merck), the samples were dried under vacuum for 12 h. The samples were coated with gold by sputtering and observed by SEM (AIS2100; Seron Technology, Uiwang-si, Gyeonggi-do, South Korea) at an acceleration voltage of 25 kV.
Surgical procedure
Forty-five adult Wistar male rats (weighing 200–250 g) (Iran University of Medical Sciences), were acclimatized. The whole process was ratified by animal protocol (IR IR.IUMS.REC.1389.2014). The animals were placed under standard condition for 1 week prior to use. The rats were randomly divided into three groups: blank defect (defect without scaffold, n = 15), blank scaffold (defect with alone scaffold, n = 15), and rBM-MSCs/scaffold (defect with cell-seeded scaffold, n = 15). The animals were anesthetized by an injection of ketamine hydrochloride (85 mg/kg; Ketara, Yuhan) and xylazine (15 mg/kg, TM Rompun; Bayer). After shaving the hair of the desired area located on the upper surface of the skull, a skin incision was created by surgical blade 12 from the nasofrontal area to the external occipital protuberance along the midsagittal suture. A critical size bone defect with a diameter of 8 mm was made on the parietal area at an equal distance between the temporal muscle and the sagittal suture with a milling machine. The scaffolds with cultured rBM-MSCs on them were then fixed within the defect and the suture line was disinfected with tetracycline.
Histology and immunohistochemistry assessments
Calvarial bone specimens were prepared as previously described (Johari et al., 2016a). For each histological block, at least three sections were studied using optical microscopy (BH-2; Olympus). Briefly, calvarial specimens were fixed in 10% buffered formalin for 12 h and decalcified in 10% EDTA for 15 days. After washing the specimens with distilled water several times, they were dehydrated with ascending concentrations of ethyl alcohol, cleared in xylene (Sigma-Aldrich), and embedded in paraffin. Serial 5 µm thick sections were obtained from the central section of each defect site using a microtome and stained with hematoxylin and eosin (H&E), Masson's trichrome staining with previously described methods (Ohgushi et al., 1989; Mumford and Simpson, 1992).
Tissue samples obtained from defects at 12 weeks post-transplantation were studied by immunohistochemistry (IHC). For COL I, a DAB detection kit (Ventana medical system) was used. All tissue samples were rehydrated by phosphate-buffered saline (PBS; Sigma-Aldrich). Unmasking antigen was performed by incubating specimens with pepsin 0.5% for 15 min. After washing the sections with PBS, endogenous peroxidase was exposed to hydrogen peroxide in PBS for 10 min. Rat primary monoclonal antibodies (dilution 1:60 antibodies; Acris), transglutaminase (TG2) II (Labvision; dilution 1:40), and the rat nonessential antibody (IgG1) were used as negative controls and incubated for a full day in 88°C. Specimens were washed by PBS, and incubated with rat's biotinylated secondary antibody (density 1:50) for 90 min in room temperature. After washing with PBS, immune localization was carried out using 3,3’-diaminobenzidine. Finally, this process was completed using nuclear hematoxylin counterstaining to reveal the cores.
Histomorphometry analysis
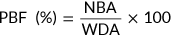 (5)
(5)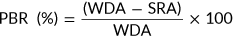 (6)
(6)Statistical analysis
Statistical analysis was performed using GraphPad Prism 6. The data rows were examined by t test and analysis of variance test, and expressed as mean ± SD. P value <0.05 was considered as the level of significance. All experiments were carried out with at least three times biological repetition with two times technical repetitions. P < 0.05(*), P < 0.01 (**), P < 0.001 (***), and P < 0.0001 (****).
Results
Characteristics of the nHA/GEL/SiC scaffold
Structural properties of the powders
XRD micrographs and TEM images of the nanocrystalline HA and SiC powders are presented in Figure 1A (a, b). The XRD analysis was performed using an X-ray diffractometer. The XRD pattern, shown in Figure 1A (a), indicated that nHA was formed in a semi-crystalline manner, and traces of other calcium phosphate impurities were not detected. The XRD pattern of the nHA samples can be completely indexed with apatite in the standard card (JCPDS No. 09–0432), the only phase found present. Similarly, the straight baseline and sharp peaks of the diffractogram confirmed that the SiC was well crystallized and perfectly matched with the SiC in the standard card (JCPDS No. 02–1048), shown in Figure 1A (b). No processing residue or secondary phases were found in the materials. Figure 1A also shows the typical TEM micrographs of the synthesized nHA and SiC. According to this figure, the average particle size of both powders was <100 nm. In addition, the particle size distribution for nHA and SiC powder was in the range of 80–120 nm and 40–80, respectively.
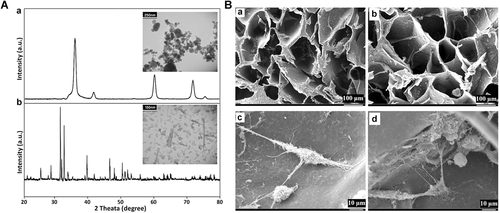
(A) X-ray diffraction (XRD) pattern and transmission electron microscope (TEM) image for the synthesized nano-hydroxyapatite (nHA) (a). XRD pattern and TEM image for the synthesized silicon carbide (SiC) (b). (B) Scanning electron microscopic micrographs of the nHA/Gel (a) and nHA/Gel/SiC (b) nanocomposites. The morphology of the cells cultured on the nHA/Gel (c) and nHA/Gel/SiC (d) nanocomposites.
Microstructure of the scaffolds
Figure 1B (a, b) shows the SEM micrographs taken from the porous surface of the scaffolds. Both nHA/Gel and nHA/GEL/SiC nanocomposites showed a homogenous microstructure with an inter-connective pore network. The pores were separated from each other by thin walls. The pores were uniformly distributed throughout the scaffolds. The porosity and density of the prepared scaffolds were determined by the liquid displacement method, as described in our previously published paper (Gholipourmalekabadi et al., 2015c), and listed in Table 1. The density of the scaffold slightly increased with SiC addition, but it was not statistically significant (independent sample t test, P > 0.05). In addition, the presence of 10% SiC did not change the porosity rate of the nHA/Gel/SiC nanocomposite in comparison to the nHA/Gel.
| Scaffolds | Natural bone | |||
|---|---|---|---|---|
| Properties | nHA/Gel | nHA/Gel/SiC | Spongy bone | Compact bone |
| Porosity(% | 83 ± 2 | 87 ± 5 | 30–90 | 5–30 |
| Average pore size (µm) | 460 ± 73 | 479 ± 57 | – | – |
| E (MPa) | 98 ± 8 | 112 ± 9 | 20–500 | 3–30 × 103 |
| σyeild(MPa) | 3.1 ± 0.7 | 3.4 ± 0.2 | 4–12 | 130–180 |
| ρ (g/cm3) | 0.33 ± 0.05 | 0.37 ± 0.09 | 0.14–1.2 | 1.8–2 |
| E/ρ | 297 ± 39 | 304 ± 13 | 142–417 | 1.78–15 × 103 |
| σ/ρ | 9.39 ± 1.5 | 9.28 ± 3 | 10–30 | 72–90 |
- E, elastic modulus; σyield, yield strength; ρ, density; E/ρ, specific (elastic) modulus; σyield/ρ, specific (yield) strength.
Besides the porosity and pore size, an ideal scaffold for bone tissue engineering requires adequate mechanical properties that mimic natural bone. According to the results obtained from mechanical evaluation of the scaffolds, addition of SiC profoundly increased the mechanical properties of the samples (Table 1).
The mechanical behavior of the prepared dry scaffolds was studied by conducting a compressive test according to ASTM F 451-86. For this, the scaffolds were cut to 6 × 12 mm. Dimensions were measured with an electric digital caliper. This test was performed using a Roel-Amstel mechanical instrument with a strain rate of 1 mm/min. Each test was repeated four times and the average amount and SD of related parameters such as E (Young's modulus) and yield strength (σ yield) were calculated.
The obtained values for mechanical indices of the prepared scaffold were in accordance with our previously published reports (Azami et al., 2010a, b, c) and in this case, addition of SiC improved the mechanical properties of these scaffolds. However, since the scaffold were tested in a dry state, higher values for mechanical indices might be obtained compared with wet samples.
Characterization of rBM-MSCs
The cells were positive for CD90, CD105, and negative for CD34, CD45 (Figure 2A). The differentiation capacity of rBM-MSCs into osteogenic and adipogenic cells were investigated under in vitro conditions. In this analysis, calcium deposition by osteoblasts and oily vesicles in adipocytes was detected with alizarin red and Oil red staining, respectively. These results indicated that the isolated rBM-MSCs are MSCs (Figure 2B).
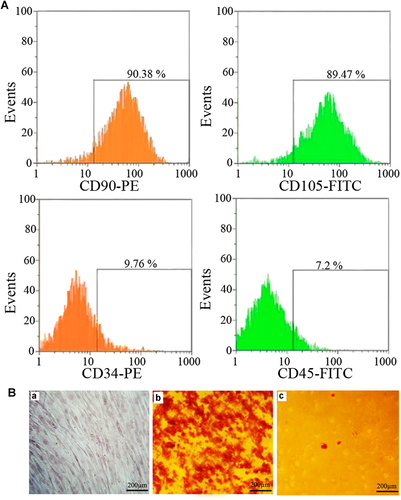
(A) The surface markers expression of mesenchymal stem cells (MSCs) was determined using flow cytometry. The cells positively expressed the surface markers CD90, CD105 while the expression of hematopoietic markers CD45 and CD34 was very low. (B) The differentiation potential of MSCs was analyzed, (a) cultured mesenchymal stem cells (b) Alizarin red, and (c) Oil red O staining of rBM-MSCs after osteogenic and adipogenic induction, respectively.
Biological behavior
The morphology of the cells cultured on the nHA/Gel and nHA/Gel/SiC nanocomposites are shown in Figure 1B (c, d), respectively. As shown in Figure 1B, the cells were actively growing, attached to both synthesized scaffolds, and migrated into the pores of the scaffolds. Expansion of the cytoplasm of the cells in all directions indicated the high biocompatibility, cell adhesion, and non-cytotoxicity properties of the scaffolds. Therefore, addition of SiC did not affect the cell adhesion property of the resulted scaffold.
The results obtained from MTT test and the LDH-specific assay are shown in Figures 3A and 3B, respectively. As can be seen, addition of 10% SiC did not change the rate of cell proliferation in all of the tested time points, when the positive control was considered as 100% cell viability. The nHA/Gel/SiC nanocomposite did not show any detectable cytotoxicity against the cells in comparison to the nHA/Gel and the positive control.
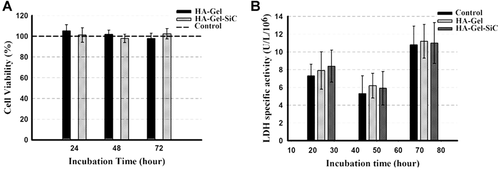
(A) 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and (B) lactate dehydrogenase (LDH) results of the nano-hydroxyapatite/gelatin (nHA/Gel) and nHA/Gel/silicon carbide (SiC) three-dimensional nanocomposites in comparison with the control. No significant differences were observed between the samples (independent sample t test, P > 0.05).
Histological and immunohistochemical evaluation
Rats were sacrificed at 1, 4, and 12 weeks post-implantation and the calvaria with a minimum amount of host bone were harvested for histological and immunohistochemical examination. Masson's Trichrome staining and immunohistochemistry COL I were used to observe formation of collagen.
After 1 week, histological results showed not significant new bone formation in the blank defect, blank scaffold and cell/scaffold groups and the scaffolds maintained their integrity. In all groups, thin connective tissue was observed with no substantial amount of collagen formation. Accumulation of multi-nucleated giant cells and inflammation cells were observed at the lesion site (Figure 4).
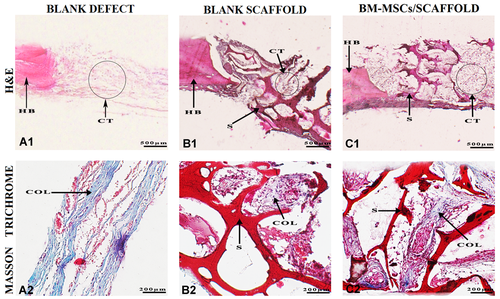
Histological assessment of the specimens harvested of rat calvarial after 1 week of post-implantation. (A1, B1, and C1) Hematoxylin and eosin (H&E) staining images showing defect and graft areas along with the host bone. (A2, B2, and C2) Masson's Trichrome staining of collagen synthesis. COL, collagen fibers; CT, connective tissue; HB, host bone; S, scaffold.
After 4 weeks, slight osteogenesis was observed on the edges of the defect and the connective tissue was more pronounced and thicker in comparison with 1 week in the blank defect group. In the blank scaffold group, bone tissue from the periphery of the defect and also the bony islands around the scaffold pores that were degraded were detectable. In the cell/scaffold group, a higher amount of bone formation was observed not only at the edges of defects but also around the degrading scaffold. Synthesis of collagen in varying densities could be observed in all three groups. At week 12, multi-nucleated giant cells or inflammatory cells were not observed around the scaffolds, indicating no cytotoxicity as a result of the scaffold destruction process (Figure 5).
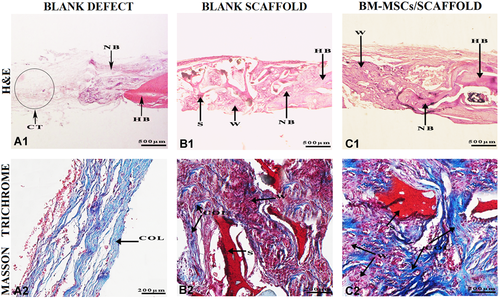
Histological assessment of the specimens harvested of rat calvarial after 4 weeks of post-implantation. (A1, B1, and C1) Hematoxylin and eosin (H&E) staining images showing defect and graft areas along with the host bone. (A2, B2, and C2) Masson's Trichrome staining of collagen synthesis. COL, collagen fibers; CT, connective tissue; HB, host bone; NB, new bone; S, scaffold; W: woven.
After 12 weeks, in the blank defect group, a small amount of bone formation was observed, which was limited to the edges of scaffold. The thickness and amount of connective tissue had increased from 1 week until 12 weeks. In both blank scaffold and cell/scaffold groups, the remnants of scaffold were barely detectable in the graft area but the defects were largely filled with bone tissue around the degraded scaffold. In the blank scaffold group, woven bone formation increased at the edge of the host bone toward the center of the graft area. Over time, the scaffold's lamellas were replaced with woven bone. In the cell-scaffold group, lamellar bone formation increased from the woven bone toward the center of defect. Scaffold destruction continued until 12 weeks, but at the end of this time, no substantial amount of scaffold remained. According to the IHC result, production of COL I in the cell-seeded scaffold group was substantially elevated in comparison with the blank scaffold and blank defect groups (Figure 6).
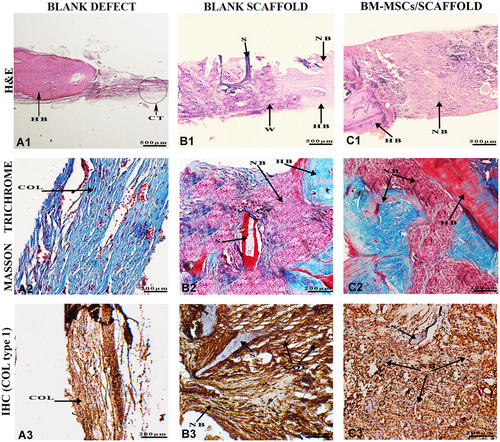
Histological and immunohistochemical evaluation of the specimens harvested of rat calvarial after 12 weeks of post-implantation. (A1, B1, and C1) Hematoxylin and eosin (H&E) staining images showing defect and graft areas along with the host bone. (A2, B2, and C2) Masson's Trichrome staining of collagen synthesis. (A3, B3, and C3) Immunohistochemical (IHC) staining for collagen type I, which significantly increased in defects with cell-seeded scaffolds compared with other defects. COL, collagen fibers; CT, connective tissue; HB, host bone; NB, new bone; S, scaffold; W: woven.
Quantitative analysis
Histomorphometric analysis indicated a significant increase in new bone formation in the cell-seeded scaffold group compared with the blank defect and blank scaffold groups (Figure 7A). In addition, the data confirms the higher biodegradation rate of the scaffold in the cell-seeded group compared with the blank scaffold group (Figure 7B).
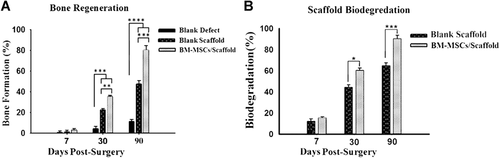
Histomorphometric analysis of (A) new bone formation and (B) biodegradation of the scaffold at 1, 4, and 12 weeks post-transplantation. All data were obtained from analysis of hematoxylin and eosin (H&E) stained samples with ImageJ software (http://imagej.nih.gov/ij/, 1997–2016). The values are expressed as the percentage of the whole area of the defect. Data were reported as mean ± standard deviation and each assay was repeated three times. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, one-way analysis of variance, P < 0.05.
Discussion
Despite favorable results of using autografts in critical bone defects, their utility has been limited by some drawbacks such as quantity limitation and prolonged recovery time. To eliminate these limitations, a great interest has developed in using nanocomposite scaffolds (Betz, 2002; Buttermann, 2008). Applying different scaffolds in bone tissue engineering is a widely used approach to induce bone formation and provide a suitable microenvironment for growth of bone cells (Laurencin et al., 2006). An ideal scaffold for applying in bone tissue engineering requires adequate porosity (at least 30%) and pore size (at least 100 µm in diameter) to facilitate cell migration, differentiation, and ingrowth within the scaffold (Okii et al., 2001; Gholipourmalekabadi et al., 2015c; Kouhi et al., 2016). Both spongy and compact bones are considered as a real composite with a different porosity and mechanical property. Therefore, the mechanical data obtained from this study was compared with those in natural bone. The results showed the porosity percentage of 83 ± 2% and 87 ± 5% for nHA/Gel and nHA/Gel/SiC nanocomposites, respectively. The data indicated that the porosity of both the developed scaffolds was comparable with those of the porosity in spongy bone (30–90%). Furthermore, no significant differences were observed in the density of the scaffolds (independent sample t test, P > 0.05). Taken together, both nHA/Gel and nHA/Gel/SiC scaffolds provided sufficient porosity and inter-connective pores with a desirable shape and size. Kim et al. (2005b) showed that the increase of the nHA ratio in the nHA/Gel construct negatively affects the morphology, porosity, and pore size of scaffold. They showed that the nHA/Gel scaffold with 10% nHA exhibits a more uniform structure with a higher porosity and pore size compared with 30% nHA. However, in the current study, the nHA/Gel scaffold with 40% nHA showed a uniform structure with no apparent difference in the morphology of the pores after adding SiC. Additionally, no significant difference in the size of the pores (Table 1) was observed in the developed scaffolds (independent sample t test, P > 0.05).
In the present study, bone marrow cells were isolated and their phenotype determined. The cells were expressing markers of MSCs (CD90, CD105), and adhered to the scaffold. Although 9.76% and 7.2% of the cells expressed CD34 and CD45 (negative markers) on their surface, the phenotypic evaluations exhibited that more than 89% of the cells were positive for CD90 and CD105, two specific CD markers for MSCs. All findings obtained in this study were consistent with other related studies (Elkhenany et al., 2015; Villa et al., 2015; Samadikuchaksaraei et al., 2016).
No detectable cytotoxicity of the nHA/Gel nanocomposites was found in our previous published study (Kim et al., 2005b). The biocompatibility of the HA, Gel and SiC has been well proven in various studies and there are no reports so far of their cytotoxicity (Munoz et al., 2002; Kim et al., 2005a; Ali Samadikuchaksaraei and Ghafouri, 2009; San Thian et al., 2006; Aminian et al., 2011). The SiC positively affected the mechanical properties, while it did not change the biocompatibility of the synthesized scaffold. The results obtained in the present study strongly suggest SiC as a favorable material for enhancing the biomechanical properties of the nHA/Gel nanocomposite for utilizing in hard tissue regeneration, especially bone.
Biocompatibility is a decisive factor in scaffolds, which affects their clinical usage. A suitable scaffold effectively stimulates the tissue repairing mechanisms and avoids any inflammatory response. Several studies have been conducted on biocompatibility of nanocomposite scaffolds. Johari et al. (2016b) reported that the bioglass/gelatin scaffold had no cytotoxicity for osteoblast cells. In addition, similar results were obtained from cytotoxicity studies of different nanocomposites on the MSCs, osteoblasts and endothelial cells (Azami et al., 2010b; Johari et al., 2016a; Samadikuchaksaraei et al., 2016; Kargozar et al., 2018).
nHA/Gel/SiC nanocomposites have all properties needed as an ideal scaffold in bone tissue engineering. The HA/Gel nanocomposite scaffold showed good cell attachment, cytocompatibility, biodegradability, and sufficient porosity with a desirable shape and size (Azami et al., 2010b; Johari et al., 2016a). About 70% of the bone structure is composed of nanocrystalline HA, which makes this substance one of the main mineral components for fabrication of a substitute, which mimics the bone structure and the microenvironment. To mimic the organic component of the bone and optimize the mechanical properties, HA is usually used in the composite form with polymeric components such as gelatin (Samadikuchaksaraei et al., 2016; Kargozar et al., 2018). In this case, another strategy was also adopted to increase the regenerative properties of the microenvironment provided by the bone scaffolds via incorporation of silicon nitride into the body of the scaffold (Munoz et al., 2002). As reported by other researchers, SiC positively affected the mechanical properties, while it did not change the biocompatibility of the synthesized scaffold and can facilitate cell migration and cellular activity through augmentation of extracellular deposition (Johari et al., 2012; Poursamar et al., 2009).
In this study, the bone regeneration potential of nHA/Gel/SiC/rBM-MSCS scaffold was investigated using the rat calvarial defect as an in vivo model. Histopathological evaluations showed that the nHA/Gel/SiC/rBM-MSCs could better improve bone repair as compared with the other groups. At the first week, thin connective tissue with a small amount of collagen was observed in the scaffold's cavities of both blank scaffold and cell/scaffold groups. After 4 weeks, as bone formation progressed, the collagen deposition increased, and bone islands were observed at the pores and center of the scaffold. At week 12, the lamellar bone near the edges and woven bone at the center of the defect grew and the defects were completely filled. In the blank defect, the connective tissue at week 4 was thicker in comparison with week 1, and the bone formation at week 4 until week 12 was only limited to the edges of the defects. The process of bone formation started at week 4 in both the blank scaffold and cell/scaffold groups, while the process began earlier in the cell/scaffold group. Moreover, the collagen deposition was significantly higher in the cell/scaffold group in comparison with the other two groups.
It was reported that the application of ceramic nanocomposites alone, did not lead to promising results in the treatment of the rat's bone defect, while cell-seeded ceramic nanocomposites showed better osteogenesis (Chen et al., 2013).
In a study conducted by Khojasteh et al. (2017) minimum amount of bone formation was reported in the blank scaffold group. In that study, adding mesenchymal cells to scaffolds improved the bone formation. The study also found that the combination of MSCs and EPCs could remarkably elevate osteogenesis and lamellar bone formation. In that investigation, maximum osteogenesis was observed in the VEGF/MSC scaffold group.
In the present study, scaffold degradation continued until 12 weeks, so that at the end of this week only a small amount remained. The degradation rate of the cell/scaffold was higher than the blank/scaffold, and overall at the end of 12 weeks, more than 90% of the scaffolds were absorbed. The scaffold degradation process observed in this study is consistent with other related studies (Gholipourmalekabadi et al., 2015c; Johari et al., 2016a; Samadikuchaksaraei et al., 2016). Initially, multi-nucleated giant cells and inflammatory cells were present at the lesion site. However, at week 4, these cells were not detected in the defect area, indicating that the scaffold degradation process was not toxic to the tissue.
Conclusions
In this study, we found that SiC had a positive effect on the mechanical behavior of the synthesized scaffold, while it did not affect its biocompatibility and ability to stimulate bone regeneration. The in vivo results showed a remarkably increased biocompatibility, bone formation, osteoinduction, and biodegradation of the rBM-MSCs/scaffold at the defect site of the rat calvarial bone. Altogether, the HA/ gelatin/SiC scaffold along with rBM-MSCs can be useful for bone repair.
Funding
This work was supported by a grant from the Iran Medical Science University as a Master of Science thesis.
Conflict of interests
The authors declare that there are no conflict of interests.




