Spinal cord tissue affects sprouting from aortic fragments in ex vivo co-culture
Abstract
It is a well-known fact, that there is a close interconnection between vascular and neural structures in both embryonic development and postnatal life. Different models have been employed to dissect the mechanisms of these interactions, ranging from in vitro systems (e.g., co-culture of neural and endothelial cells) to in vivo imaging of central neural system recovery in laboratory animals after artificially induced trauma. Nevertheless, most of these models have serious limitations. Here, we describe an ex vivo model, representing an organotypic co-culture of aortic fragments (AF) with longitudinal slices of mouse neonatal spinal cord (SC) or dorsal root ganglia (DRG). The samples were co-cultured in a medium adapted for SC tissue and lacking any pro-angiogenic or neurotrophic growth factors. It was found, that cultivation of AFs in the SC injury zone (transversal dissection of a SC slice) resulted in the initiation of active aortic sprouting. Remarkably, the endothelial cells exiting the AFs never invaded the SC tissue, concentrating in a nearby area (negative taxis). In contrast, the DRGs, while also promoting the sprouting, were a target of active endothelial CD31+ cell invasion (positive taxis). Thus, the tissues of both central and peripheral nervous systems have a prominent positive effect on aortic sprouting, while the vector of endothelial cell expansion is strictly nervous-tissue-type dependent. The ex vivo AF co-culture with SC or DRG appeared to be a useful and promising model for a further endeavor into the mechanisms driving the complex interactions between neural and endothelial tissues.
Abbreviations
-
- AF
-
- aortic fragment
-
- DRG
-
- dorsal root ganglia
-
- PBS
-
- phosphate-buffered saline
-
- SC
-
- spinal cord
-
- SCI
-
- spinal cord injury
Introduction
Neural and vascular systems are intimately linked in both embryonic development and postnatal functioning. A substantial line of evidence confirms the effects of vascular structures upon neuronal growth. It was shown that motor and sensory neurons permeate embryonic skin only after the formation of a primary capillary network at embryonic stage E13.5 (James and Mukouyama, 2011). Blood vessels also have prominent effects on differentiation of neuronal precursors and on axonal growth—co-cultivation of the subventricular zone explants with endothelial cells stimulates migration of neuronal precursors from the explants and neurite outgrowth (Leventhal et al., 1999). In turn, nerves have their effects upon blood vessels as well: death or reorientation of peripheral nerves in embryonic mouse skin disrupts the differentiation of endothelial precursors into artery cells and alter the direction of vascular growth (Mukouyama et al., 2002). Active interaction of neural and endothelial cells was demonstrated not only in physiological settings, but in pathology as well. It was shown, that VEGF-A, secreted by macrophages in damaged mouse sciatic nerves, induces oriented growth of new blood vessels which eventually serve as ‘tracks’ for Schwann cell migration (Cattin et al., 2015). It should be noted, however, that the process of vessel remodeling after the injury is not the same in small capillaries (angiogenesis) and in arteries (arteriogenesis). Angiogenesis is driven by hypoxia and HIF pathway activation leading to induction of a number of pro-angiogenic factors such as VEGF, FGF, TGFb, and so on. These effects promote the proliferation and migration of endothelial cells (Carmeliet, 2000; Ucuzian et al., 2010). In contrast, arteriogenesis is induced primarily by mechanical stress, for example, by alteration of shear forces due to the shifts in blood pressure. This process is driven mostly by genes bearing shear stress responsive elements and is oxygen independent (Resnick et al., 2003). Therefore, given these differences, small and large vessels may respond differently to paracrine injury signaling not only in pathology but in an experimental setting as well. Furthermore, given the extensive innervation of large vessels (Sheng and Zhu, 2018), they innately possess appropriate sensory mechanisms and thus may be more sensitive to paracrine neural signaling as compared with capillaries.
Currently, the interactions between neural and vascular tissues are studied mostly in vitro using co-culture of neural and endothelial cells (Xue et al., 2013). As an occasional in vivo alternative, lab rodent models are used in combination with immunohistochemistry (Benton et al., 2009) or in vivo imaging (Dray et al., 2009) as methods aimed at assessment of the damage and regeneration of injured neural tissues. Nevertheless, for studies of molecular and cellular aspects of extensive neural tissue damage [e.g., spinal cord injury (SCI)], the use of lab animals is complicated by several problems. Among them, the necessity to enlist the help of a surgeon, complicated post-operative animal care and high cost of experiments. But most importantly, in vivo experiments involving neural tissue damage and regeneration have low reproducibility and usually require large numbers of animals to compensate for this problem.
In the current study, we used organotypic co-cultures of mouse aortic fragments (AF) with either longitudinal slices of mouse spinal cord (SC) or isolated dorsal root ganglia (DRG) to assess the neural-endothelial interactions. The SC slices were cut transversely to mimic SCI. The culturing of AFs in the zone of SC transection (the injury site) prompted active sprouting of the aorta, while, if AFs were cultivated to the side of the SC slices (not in the injury zone), the sprouting was observed only in a minor fraction of samples. Surprisingly, the endothelial cells exiting the AFs never migrated into the SC slice, but rather concentrated in the injury zone free of neural tissue. Co-cultivation of AFs with DRGs, isolated from the same newborn mice as SC slices, also resulted in activation of aortic sprouting. However, in striking contrast to co-cultivation with SC slices, the endothelial cells actively invaded the ganglia.
Based on the study, we propose a new ex vivo organotypic culture system, which models and illustrates the interactions between neural tissue (SC) and vascular structures in vivo. Using this model, we demonstrate that the cells of both the central and peripheral nervous systems can stimulate endothelial sprouting. At the same time, the direction of endothelial cell migration, as well as the ability of these cells to invade neural tissue, is strictly dependent on neural tissue type.
Materials and methods
Organotypic culture
All animal procedures were carried out in strict adherence to the requirements of the Local Ethics Committee on Biomedical Research (NRC ‘Kurchatov Institute’; Protocol #5, April 5, 2017). C57BL/6J newborn mouse pups (P1–P6) were promptly decapitated. SCs were dissected out and placed in ice-cold Leibovitz's L-15 medium (Gibco, USA) with 6.5 mg/mL d-glucose (Helicon, Russia) and penicillin/streptomycin, 50 U/mL and 50 μg/mL, respectively (Paneco, Russia). The meninges were removed and the thoracolumbar region of the SC was rapidly transferred onto McIlwain tissue chopper using a plastic Pasteur pipette. Longitudinal slices were cut at a thickness of 350 µm and placed onto a Millicell (Millicell, Germany) culture insert membrane pre-coated with 70–150 kDa poly-l-lysine (Sigma, USA) at 0.1 mg/mL. A transverse cut was performed using a scalpel blade. The average size of the ‘injury zone’ (the distance between split SC slices) was 900 ± 300 µm.
DRGs were isolated from the same pups as the SC and placed in L-15 medium. Most of the remaining neural fragments were removed but some preparations still contain remnants of peripheral nerves (an arrow on Figure 4D’). The DRGs were then transferred onto the Millicell culture insert membranes, similarly to SC preparations.
Aortas were obtained from the same pups as neural tissues. The aortic adventitia was carefully removed by gentle peeling with fine forceps under a dissecting microscope, and aortas were dissected into cylindrical fragments (about 1 mm long) using a scalpel. Isolated AFs were arranged in three different ways: (1) next to the SC slice (Figure 1A), such that the AF was touching the lateral side of the slice or one of its ends; (2) precisely in the injury zone (Figure 1B), between the two dissected fragments of the SC slice; (3) between two DRGs, such that the AF was touching both ganglia.
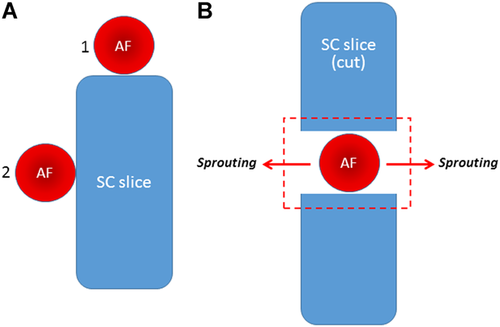
The schematics showing the arrangement of aortic fragments (AF) and spinal cord (SC) slices in different experimental settings. (A) AF placement near the end of the SC slice (1) or at the side of the SC (2). (B) AF placement in the zone of SC transection (the injury zone—red dashed rectangle) augments lateral AF sprouting (arrows).
Organotypic cultures (in all combinations) were maintained at 5% CO2 and 37°C in a medium composed of 50% MEM with glutamine (Sigma), 25% Hank's Balanced Salt Solution (Paneco), 25% heat-inactivated horse serum (Gibco), 0.65% glucose and penicillin/streptomycin (50 U/mL and 50 μg/mL, respectively). The medium was changed every second day. Cultures were incubated for 5–7 days.
Immunohistochemistry
Organotypic cultures were washed in phosphate-buffered saline (PBS) and fixed with formaldehyde (3.8%, 45 min, room temperature; PanReac, Germany). After that, cultures were washed three times for 10 min with PBS and SC slices with aorta or SC slices with DRG were cut off with the PTFE membrane and blocked in a 24-well plate containing PBS with 0.3% Triton X-100 and 8% normal donkey serum (Sigma) for 60 min. Then cultures were incubated (24 h, +4°C) with the following primary antibodies: β3-tubulin (mouse, 1:300; Abcam; ab78078) and CD31 (rabbit, 1:50; Abcam; ab 28364). Sections were then washed three times for 10 min with PBS and incubated with the secondary antibodies: donkey anti-mouse IgG H&L (Cy™3, 1:800; Jackson ImmunoResearch; 715-166-150) and donkey anti-rabbit IgG H&L (Alexa Fluor® 488, 1:800; Jackson ImmunoResearch; 711-546-152) for 2.5 h at room temperature. Following PBS washes, cultures were counterstained with DAPI (1 µg/mL) and mounted using Immu-mount (Thermo Fisher Scientific, USA). Samples were observed under a fluorescent microscope (Zeiss). Images were acquired with an AxioCam MRm camera (Zeiss) using Axiovision 4.8 software. In some cases, when the sample did not fit into the field of view, the final photograph was compiled from several images (Figures 2C, 3A, 3C, 5A, and 5B).

Culturing of aortic fragments (AFs) next to spinal cord (SC) slices (5–7 days of co-culture). In 77% of samples endothelial cell migration from AFs (sprouting) was not observed (A). In the remaining 23% of samples migration of CD31+ cells from the AFs was evident (B, C). Asterisk demarcates the zone with obvious orientation of CD31+ cell migration away from the SC slices (C). CD31+ cells were always confined to the zones occupied by other (CD31−) AF-derived cells (D). On (B) and (D) the same sample is shown. CD31: green; β3-tubulin: orange; DAPI: blue. Scale bar = 200 µm.
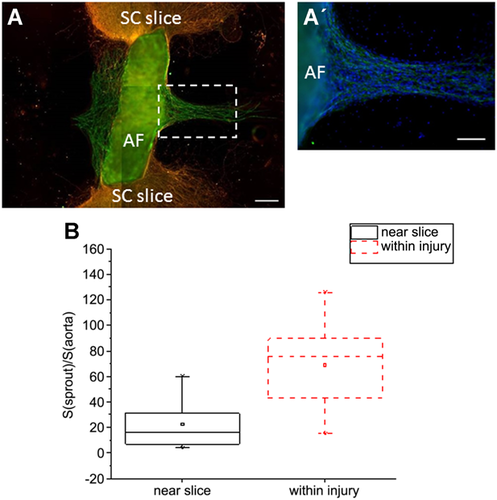
Culturing of aortic fragments (AFs) in the zone of spinal cord (SC) injury. (A) CD31+ cells migrate from AFs to the zone of SC injury but do not invade the SC tissue itself. (A’) Blow-up of the framed (white dashed rectangle) zone on (A): CD31+ cells are located exclusively in the region occupied by other (CD31−) AF-derived cell types. (B) Quantification of sprouting intensity; statistical significance was determined using the Mann–Whitney test; P = 0.011. CD31: green; β3-tubulin: orange; DAPI: blue. Scale bar = 200 µm (A) or 100 µm (A’).
Sprouting assessment and statistics
The intensity of sprouting was assessed using ImageJ (an open source image processing program; https://imagej.nih.gov/ij/index.html) with the correlation coefficient K representing the ratio (%) of the area occupied by CD31+ endothelial emigres to the area of the AF itself. OriginPro 2016 software was used for statistical analysis of the data. The Mann–Whitney test was performed to evaluate the statistical differences. All experiments were repeated four to five times.
Results and discussion
It should be mentioned at the outset that joint cultivation of SC slices and AFs was carried out in the medium adopted for ex vivo culturing of SC tissue. Thus, the medium was lacking angiogenesis-stimulating growth factors such as VEGF, bFGF, and others (Bouïs et al., 2006). In this medium, aortic cell migration (including CD31+ cells) was never observed in the absence of SC slices.
AF cultivation next to SC slices
After 5–7 days of AFs cultivation at the side of the SC slices, only 23% of AFs showed the migration of CD31+ endothelial cells (Figures 2B and 2C), while in most samples (77% of AFs) aortic sprouting was not observed (Figure 2A). As seen from Figure 2D, CD31+ cells comprised only a small fraction of cells exiting the AFs and were always found among other migrating cells, never forming their own, exclusively ‘endothelial’ route of migration. Notably, these endothelial (CD31+) cells never invaded the SC tissue and were predominantly situated in the SC-free area (Figures 2B and 2C). It is evident from Figure 2C that the endothelial cells from AF (zone*) migrate strictly away from the SC slice. Thus, this type of AF/SC relative positioning induced sprouting in 23% of AFs despite the absence of pro-angiogenic growth factors in the culture medium.
AF cultivation in the zone of SC injury
In another series of the experiments, the AFs were cultivated for 5–7 days directly between the two parts of the transected SC slices (within the ‘injury zone,’ see red square on Figure 1B). Such positioning of AFs resulted in significant activation of endothelial cell migration. Migration of CD31+ cells was observed in 75% of the AFs in contrast to 23% of AFs placed at the side of the SC. Moreover, the area occupied by these CD31+ cells migrating into the injury zone was larger as compared with the first series of the experiments (compare Figure 3A with Figure 2B). The intensity of sprouting, denoted by coefficient K (the ratio of the area occupied by exiting CD31+ cells to the area occupied by the AF itself, %), was also higher for AFs cultivated in the injury zone as compared with aortas positioned at the side of the SC (mean K was 69 and 23%, respectively; see also Figure 3B). These findings suggest that the concentration of growth factors that support endothelial cell survival, proliferation, and migration is much higher in the SC injury zone. Previously it was shown that along with the well-recognized pro-angiogenic factor VEGF, survival, proliferation, and migration of endothelial cells is also regulated by such neurotrophic factors as BDNF and NGF (Dollé et al., 2005; Kermani and Hempstead, 2007). It is well known that SC trauma results in significant upregulation of multiple growth factors at the immediate site of the injury, including VEGF, BDNF, and NGF (Dougherty et al., 2000; Krenz and Weaver, 2000; Sköld et al., 2000; Graumann et al., 2011). Given all of the above, we assume that intensification of aortic sprouting in the injury zone is linked to locally increased concentration of some (or all) of these factors. At the sides of the SC slices, the concentration of these factors does not seem to be sufficient to induce active sprouting. These suggestions require further validation on qualitative (the list of factors secreted by SC stumps and inducing AF sprouting) and quantitative (extent of induction) levels.
In addition to AFs, we also accomplished a series of experiments using fragments of mouse vena cava. Migration of endothelial cells from vena cava was much less intensive as compared with aorta. Therefore, the results obtained with vena cava were not taken into consideration and are not shown here. Nevertheless, the differential sprouting response (in the presence of damaged neural tissue) in vessels of the different type still remain a possibility and should be checked specifically.
The vector of CD31+ cell migration
The migration of CD31+ cells was observed exclusively in the range of localization of other AF-derived (CD31−) cells in both types of experiments (AF placement at the side of SC slice and in the injury zone) (Figures 2B and 3A’). We did not evaluate the nature of these CD31− cells, but apparently, fibroblasts represented at least part of them. First, it is known that fibroblasts and macrophages are the first line of cells migrating from the AFs cultured in a gel-like medium (Nicosia, 2009). Second, fibroblasts are the chief producers of extracellular matrix components and growth factors, required for angiogenesis support (Martin et al., 1999; Newman et al., 2011). Both these factors taken together may explain the presence of CD31+ cells specifically in the zone of fibroblast migration.
Importantly, while AF-derived CD31+ cells filled in the injury zone, they never permeated the SC tissue itself (Figure 3A). Furthermore, endothelial outgrowths were occasionally oriented perpendicularly to the SC slice axis—in the directions most diverted from the SC tissue (Figures 1B and 3A). These observations prompt to suggest that, along with angiogenesis-stimulating factors, the damaged SC tissue also secretes factors acting to repel the AF sprouts (a negative taxis). This suggestion is in line with recent findings that during the embryonic development of the SC, the motor neuron column remains non-vascularized until stage Е12.5, despite high levels of VEGF expression. It turned out that motor neurons synthesize a soluble (secreted) form of VEGF receptor-1—sFlt1 (Himmels et al., 2017). This alternatively spliced variant of VEGFR-1 serves as a decoy receptor, which sequesters VEGF from VEGFR-2 binding thus blocking VEGF activity—an effect particularly important during embryonic vasculogenesis (Zygmunt et al., 2011). The studies by Himmels et al. (2017) have also shown that motor neuron-containing neural tube explants suppress migration of endothelial cells, most likely due to active sFlt1 secretion. Similar mechanisms might be implicated in the absence of endothelial cell invasion into the SC tissue demonstrated in our experiments.
Altogether, our study provides unequivocal evidence that SC injury is sufficient to induce aortic sprouting in an x vivo system even without supplementation with any pro-angiogenic factors.
AF cultivation with dorsal ganglia
Given that the above experiments clearly confirmed the presence of stimulatory effects of the central nervous system (i.e., SC tissue) upon aortic sprouting, we further assessed whether the peripheral nervous system (i.e., DRGs alone or with the remnants of peripheral nerves) is capable of the same influence upon endothelial outgrowth. We first confirmed that DRGs can be maintained at SC-adapted culture conditions at least for 5–7 days and can produce neurites in this experimental setting. As evident from Figure 4, neurites are formed by the DRG cultured on both glass (A) and commercial inserts (B), while the intensity of neurite formation on glass is much higher. Hence, the selected culture conditions appeared to be sufficient for the experiments with DRG.
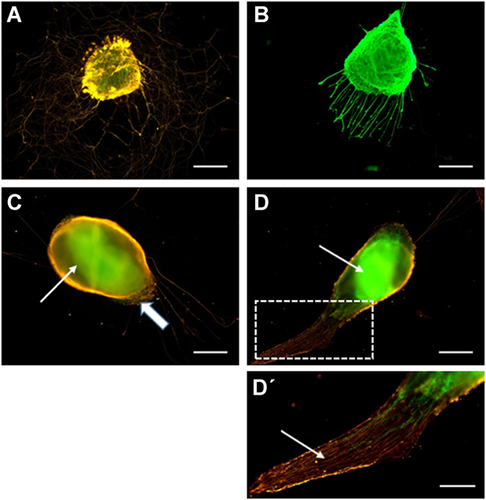
Culturing of aortic fragment (AFs) with dorsal root ganglia. Cultivation of isolated ganglia in the medium adapted to spinal cord (SC) culture was sufficient to support neurite outgrowths on both the glass (A) and commercial inserts (B). After 5–7 days of AF cultivation with DRG, their fusion was evident [a part of the dorsal root ganglia (DRG) covering the AF tissue is marked by a block arrow]; most samples were not suitable for tracking of endothelial cell migration from the AFs (C). Despite their fusion, about 43% of samples were acceptable to observe endothelial cell migration from the AFs into the DRG tissue (D). Arrows on (C) and (D) point to AF parts fused with DRGs. (D’) Blow-up of the framed zone on (D); the arrow points to the remnants of the peripheral nerve. СD31: green; β3-tubulin: orange (A, C, D, D’) or green (b). Scale bar = 200 µm (A–D) or 100 µm (D’).
To assess DRG effects upon aortic sprouting, the AFs were cultured between two DRG for 5–7 days. Unfortunately, in most cases (57%) the AFs had shrank and fused together with the DRG (Figure 4C). Presumably, the shrinking of the AFs was driven by certain factors (specifically produced by peripheral nerves) which induce contraction of smooth muscle cells of the aortic wall (Fisher, 2010; Karimi and Milewicz, 2016). This aortic contraction usually took place at days 4–5 of joint DRG/AF cultivation and lead to a collapse of the entire system and formation of an amorphous tissue conglomerate, not suitable for any analysis of AF/DRG interactions (Figure 4C). Nevertheless, in the remaining 43% of cases, despite the AF/DRG fusion, these samples were acceptable to see the invasion of CD31+ cells into the DRG or into DRG-associated parts of a peripheral nerve (Figures 4D and 4D’). In part of these samples, the fusion of AFs and DRG was incomplete, allowing to observe the migration of CD31+ endothelial cells from the AFs. Thus, similarly to SC slices, the presence of DRG in the culture system was sufficient to induce AF sprouting (Figure 5A). However, in striking contrast with experiments involving the SC slices, the CD31+ cells migrated exclusively into the DRG (positive taxis, Figure 5A). It is noteworthy that the migration vectors of CD31− (non-endothelial) cells exiting the AFs were also highly DRG dependent, resulting in their active invasion into the DRG. Therefore, the zones adjacent to AF remained cell free (asterisks in Figure 5B) despite the active AF sprouting. These results provide further evidence of active interaction between peripheral nerves and vascular system. Previously it was shown that in the skin of mouse embryos, blood vessels are primarily formed and grow along the peripheral nerves. This association, at least in part, is supported by active VEGF secretion by neurons and Schwann cells (Mukouyama et al., 2002). Postnatally, the DRG express a wide range of growth factors, including VEGF (Sondell and Kanje, 2001), BDNF (Wetmore and Olson, 1995), and TGF-ɑ (Grotendorst et al., 1989; Xian and Zhou, 1999). All of these factors are known to affect migration of endothelial cells, suggesting that their expression in the DRG is one of the reasons why CD31+ cells preferably invade DRG tissue. Alternatively, the absence of CD31+ endothelial cells in the immediate proximity to the ganglia can be explained by the absence of the other (CD31−) AF-derived cells in this zone, which also migrate exclusively into the DRG tissue. This explanation can be validated if the ‘priming’ of the extracellular space by AF-derived cells of non-endothelial origin is proven to be essential for the migration of CD31+ cells. The exact mechanisms of DRG influence upon AF sprouting and endothelial cell migration remain to be determined.
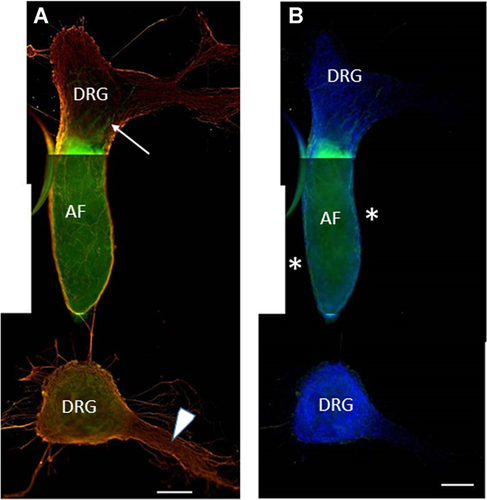
Culturing of the aortic fragment (AF) between two dorsal root ganglias (DRGs); the AF is fused with the upper DRG. (A) The lower DRG is associated with a remnant of peripheral nerve (arrowhead). In the presence of DRG, AF-derived CD31+ cells migrate inside of the DRG which is fused with the AF (an arrow points to the migrating endothelial cells). (B) Same image showing DAPI staining. Neither CD31+ nor CD31− cells are visible at the sides of the AF (*).CD31: green; β3-tubulin: orange; DAPI: blue. Scale bar = 200 µm.
Another point of remaining uncertainty is the possible difference in response to neural signaling between large and small (capillaries) vessels which possess distinct mechanisms of repair (see the ‘Introduction’ section) and therefore may respond differentially to paracrine signals emanating from neighboring tissues. SC blood supply is maintained by anterior spinal artery and two posterior spinal arteries which form a complicated network (‘the mesh’) of capillaries penetrating both, gray (more actively) and white matters (Melissano et al., 2015). The restoration of the capillary system after SC and peripheral nerves injury is relatively well-studied in vivo (Dray et al., 2009; Figley et al., 2014; Lim et al., 2015), while potential effects of neural tissue injury upon larger vessels remain undefined. Our organotypic experimental model shed some light upon this issue.
Altogether, the use of the described organotypic models has shown that fragments of both, the central and peripheral nervous systems, are capable of inducing active aortic sprouting. However, the direction of endothelial cells growth and migration is highly dependent on the type of nervous tissue. The tissues of the central nervous system (i.e., the SC) promote endothelial sprouting exclusively into the zone free of the said tissues (negative taxis), while the peripheral nervous system (i.e., DRG), on the contrary, promotes CD31+ cell invasion exclusively into its own tissues (positive taxis). These differences are presumably linked to differential expression of pro-angiogenic factors by the two types of neural tissue. The list of these factors and the exact patterns of their expression in our ex vivo system remain to be determined. However, our studies suggest that the ex vivo co-culture system comprising AFs and SC/DRG tissues represents a valid model for a further endeavor into the complex interactions between the vasculature and the central or peripheral neural tissues.
Acknowledgments and funding
The authors are grateful to Dr. A.P. Bolshakov for critical reading of the manuscript. The study was supported by an intramural grant from the NRC Kurchatov Institute and partially by the grant from the Russian Science Foundation (No. 16-15-00243).
Conflict of interest
The authors have no conflict of interest to declare.




