GEN-27 exhibits anti-inflammatory effects by suppressing the activation of NLRP3 inflammasome and NF-κB pathway
Miao Hu and Xiangping Li contributed equally to this work.
Abstract
Prolonged inflammation and deregulated cytokine production are associated with diversified inflammatory diseases. Genistein (GEN), the active and predominant isoflavonoid in dietary soybean, possesses anti-inflammatory activity. Our study aimed to assess the anti-inflammatory effects of GEN-27, a derivative of GEN, as well as explore the potential molecular mechanisms using lipopolysaccharide (LPS)-induced RAW264.7 cells. In our study, we demonstrated that GEN-27 administration (1, 5, or 10 μM) dose-dependently inhibited nitrite and nitric oxide (NO) levels in LPS-stimulated RAW264.7 cells. Also, GEN-27 suppressed the release of LPS-induced pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, and IL-18. Moreover, GEN-27 attenuated LPS-induced inducible NO synthase (iNOS), and cyclooxygenase-2 (COX-2) expressions at messenger RNA and protein levels, and reversed the promoter activity of iNOS in RAW264.7 cells. Mechanistically, GEN-27 abated LPS-induced reactive oxygen species production, as well as mitigated LPS-induced increase of caspase 1 activity and the protein levels of NOD-like receptor 3 (NLRP3), anti-apoptosis-associated speck-like protein-containing a CRAD (ASC), and caspase 1 in RAW264.7 cells in a dose-dependent manner. Similarly, GEN-27 dose-dependently weakened adenosine triphosphate-induced NLRP3 and IL-1β in RAW264.7 cells. In addition, GEN-27 treatment significantly suppressed LPS-induced phosphorylation of nuclear factor-κB (NF-κB) p65 and alleviated LPS-induced increase of transcriptional activity of NF-κB in RAW264.7 cells. In summary, these results revealed that GEN-27 exhibited anti-inflammatory effects by suppressing the activation of NLRP3 inflammasome and NF-κB pathway, suggesting that GEN-27 may be served as a promising therapeutic agent for the prevention and therapy of inflammatory-associated diseases.
Introduction
Inflammation is an important host-defense response of organisms against many destructive stimuli, including damaged cells, invading pathogens, or endotoxins (Cheng et al., 2015). Bacterial lipopolysaccharide (LPS), a potent endotoxin, is the vital biological ingredient of Gram-negative bacteria cell wall and has been well-documented to be a crucial trigger of inflammation due to its ability to activate macrophages (Gioannini and Weiss, 2007). Macrophages, the primary immune cells acting as the first line of host defense against invading pathogens, have a cardinal role in the initiation, maintenance, and resolution of inflammation. Excessively activated macrophages usually trigger excessive or uncontrolled production of inflammatory mediators, such as tumor necrosis factor-α (TNF-α), inducible nitric oxide synthase (iNOS), nitric oxide (NO), cyclooxygenase-2 (COX-2), interleukin-1β (IL-1β), and IL-6 (Cheon et al., 2015). Prolonged inflammation and deregulated cytokine production have been proposed to be associated with the pathological processes of diversified inflammatory diseases, such as rheumatoid arthritis, sepsis, and inflammatory bowel disease (Fichtner-Feigl et al., 2005, Akaogi et al., 2006, Shin et al., 2012). Therefore, LPS-induced RAW264.7 cells have been generally used as a suitable macrophage model for exploring inflammation-related mechanisms and anti-inflammation candidate screening (Chu et al., 2016).
Genistein (GEN, 4′,5,7-trihydroxyisoflavone), the active and predominant isoflavonoid in dietary soybean existing at concentrations ranging from 1.9 to 229 mg/g, has received wide attention due to its beneficial effects on human health. Due to its similar chemical structure to human estrogen, GEN possesses the estrogenic activity to attach the estrogen receptors (Glazier and Bowman, 2001). In addition, GEN, as tyrosine-specific protein kinase inhibitor, has shown to present multiple biological activities, such as anti-proliferative, pro-apoptotic, anti-oxidant, anti-inflammatory, and anti-cancer properties (Spagnuolo et al., 2015). Moreover, treatment of GEN exerts anti-inflammatory effect on high-fat diet-induced nonalcoholic steatohepatitis (NASH) rats (Ji et al., 2011). GEN-27, a newly synthesized derivative of GEN, has been reported to inhibit inflammation-induced proliferation in human colorectal cancer cells (Wang et al., 2016) as well as prevent the development of colitis-associated colorectal cancer cells (Du et al., 2016). However, the anti-inflammatory effects of GEN-27 on LPS-induced inflammation and its molecular mechanisms are not fully understood.
In our study, we explored the effects of GEN-27 on inflammatory response in LPS-stimulated RAW264.7 cells and further analyzed the effects of GEN-27 on the NOD-like receptor 3 (NLRP3) inflammasome and nuclear factor-κB (NF-κB) pathway.
Materials and methods
Cell culture and treatment
RAW264.7 cells were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and grown in Dulbecco's modified Eagle's medium (DMEM; Hyclone, Logan, UT, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin, 100 mg/mL streptomycin (Invitrogen, Carlsbad, CA, USA) at 37°C in a humidified atmosphere with 5% CO2. To stimulate the cells, the medium was exchanged for fresh DMEM containing 500 ng/mL of LPS.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
RAW264.7 cells were seeded into 96-well plates at a density of 2 × 104 cells per well and then treated with different concentrations of GEN-27 (1, 5, 10, 20 μM) (purity >99.5%; Organic Chemistry Laboratory of China Pharmaceutical University, Nanjing, China) for 1 h, followed by stimulation with or without LPS for another 12 or 24 h. Untreated RAW264.7 cells were used as a control. Subsequently, MTT solution (0.5 mg/mL; Sigma-Aldrich, St. Louis, MO, USA) was added to each well and incubated for 4 h at 37°C. Then, the culture medium was removed and 200 μL of dimethyl sulfoxide (DMSO; Sigma-Aldrich) was added to dissolve the formazan dye. The absorbance at 490 nm was recorded by using a Microplate Reader (Infinite® F200; Tecan Group Ltd., Maennedorf, Switzerland).
Determination of nitrite and NO
RAW264.7 cells were plated in 24-well plates at a density of 5 × 105 cells per well overnight and pretreated with GEN-27 (1, 5, 10 μM) for 1 h prior to stimulation with LPS (1 μg/mL) for 24 h. In brief, 100 μL of Griess reagent was mixed with an equal amount of culture supernatant and then the mixture was incubated for 15 min. The absorbance at 540 nm were determined using a Microplate Reader. The nitrite levels in culture supernatant were calculated as an indicator of NO production according to a sodium nitrite standard curve.
Treated RAW264.7 cells were collected and loaded with 1 μM fluorescent NO indicator 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM diacetate) (Molecular probes, Eugene, OR, USA) for 1 h in the dark. DAF fluorescence (excitation 492 nm, emission 515 nm) was determined by a fluorescence microscope (FV1000; Olympus, Tokyo, Japan).
Transient transfections and luciferase assays
RAW264.7 cells were seeded in 24-well plates and transiently transfected with 1 μg/mL pGL3 empty plasmids, or the indicated genes were harbored including iNOS promoter (iNOS-Luc; Bioworld, Nanjing, China) or NF-κB promoter (NF-κB-Luc; Biomyx Technology, San Diego, CA, USA), along with 0.25 μg/mL pRL-TK plasmid (as an internal control) using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. After 24 h of transfection, the cells were treated with GEN-27 (1, 5, 10 μM) for 1 h, followed by stimulation with 1 μg/mL LPS for a further 24 h. Cell lysates were collected and luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) according to the manufacturer's instruction.
Inflammatory cytokine detection
The RAW264.7 cells were treated as above and the concentrations of pro-inflammatory cytokines including IL-6, TNF-α, IL-1β, and IL-18 in the cell supernatant were determined using corresponding commercial enzyme-linked immunosorbent assay (ELISA) kits (BioLegend, Cambridge, UK).
Measurement of intracellular reactive oxygen species (ROS)
RAW264.7 cells were cultured in a six-well plate at a density of 2 × 106 per well, and treated as above. Then, the cells were harvested, washed twice with PBS, and incubated with 20 μM 2,7-dichlorofluorescein diacetate (DCFH-DA; BestBio, Shanghai, China) at 37°C for 30 h in the dark. Then cells were washed twice with cold PBS to rinse the unconjugated dye and DCF fluorescence distribution was monitored by a fluorescence microscope at an excitation wavelength of 488 nm and an emission wavelength of 525 nm.
Western blot analysis
RAW264.7 cells were treated with GEN-27 (1, 5, 10 μM) for 1 h, followed by stimulation with 1 μg/mL LPS or 5 mM adenosine triphosphate (ATP) for 24 h. Then, cells were collected and lysed with radioimmunoprecipitation lysis buffer (Beyotime, Shanghai, China) supplemented with protease inhibitor cocktail (Roche Applied Science, Penzberg, Germany). An equal amount of protein samples (20 µg/lane) was separated on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Hercules, CA, USA). Non-specific sites on the membrane were blocked with 5% non-fat milk in Tris-buffered saline (TBS) containing 0.1% Tween 20 (TBST) buffer for 2 h at room temperature and the membranes were immunoblotted with primary antibodies including anti-iNOS (1:1,000 dilution; Cell Signaling Technology, Danvers, MA, USA), anti-COX-2 (1:1,000 dilution; Cell Signaling Technology), anti-NLRP3 (1:2,000 dilution; Cell Signaling Technology), anti-apoptosis-associated speck-like protein-containing a CRAD (ASC) (1:2,000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-caspase 1 (1:1,000 dilution; Cell Signaling Technology), ani-IL-1β (1:1,000 dilution; Cell Signaling Technology), anti-phosphorylated p65 (p-p65, 1:1,000 dilution; Cell Signaling Technology), anti-p65 (1:1,000 dilution; Cell Signaling Technology), or anti-β-actin (1:2,000 dilution; Abcam, Cambridge, MA, USA) at 4°C overnight, followed by incubation with corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies (1:2,000 dilution; Abcam) at room temperature for 1 h. The protein bands were then visualized using enhanced chemiluminescence reagent (GE Healthcare Life Sciences, Little Chalfont, UK).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from the treated RAW264.7 cells using the TRIzol reagent (Invitrogen). Complementary DNA was synthesized from 1 μg of total RNA using the PrimeScript RT Reagent kit (Takara Bio Inc., Otsu, Japan). Expressions of iNOS and COX-2 were determined using Fast SYBR Green Master Mix (Thermo Fisher Scientific, Waltham, MA, USA) on an ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, California, USA), with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an endogenous control. The primers used were as follows: iNOS, forward, 5′-TTGTGCATCGACCTAGGCTGGAA-3′ and reverse, 5′-GACCTTTCGCATTAGCATGGAAGC-3′; COX-2, forward, 5′-GAAGTCTTTGGTCTGGTGCCTG-3′ and reverse, 5′-GTCTGCTGGTTTGGAATAGTTGC-3′; GAPDH, forward, 5′-GGATGCAGGGATGATGTTC-3′ and reverse, 5′-TGCACCACCAACTGCTTAG-3′. The relative gene expression was calculated using 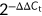 method.
method.
Caspase 1 activity assay
RAW264.7 cells treated as above were harvested and homogenized with PBS in microcentrifuge tubes. The caspase 1 activity in cell supernatant was determined using a commercial assay kit (R&D Systems, Minneapolis, MN, USA).
Statistical analysis
Data were expressed as means ± standard deviation from three repeated experiments. Significant differences were performed by Student's t test or one-way analysis of variance. All statistical analyses were carried out using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered to be statistically significant.
Results
GEN-27 exhibited anti-inflammatory effects in LPS-stimulated RAW264.7 cells
As shown in Figure 1A, no cytotoxicity was observed with GEN-27 treatment at 1, 5, or 10 μM in RAW264.7 cells with or without LPS stimulation, as demonstrated by MTT assay. However, 20 μM GEN-27 was found to be toxic for RAW264.7 cells. Therefore, non-toxic concentrations were used for further analyses. To explore the anti-inflammatory effects of GEN-27 on LPS-induced inflammation, we detected the levels of inflammatory mediators including nitrite and NO in RAW264.7 cells treated with GEN-27 (1, 5, or 10 μM) for 1 h, followed by 1 μg/mL LPS stimulation. The results demonstrated that GEN-27 dramatically reduced LPS-induced nitrite level in RAW264.7 cells in a dose-dependent manner (Figure 1B). Similarly, NO level was also dose-dependently inhibited following GEN-27 administration in LPS-stimulated RAW264.7 cells (Figure 1C). These results demonstrated that GEN-27 exhibited anti-inflammatory effects in LPS-stimulated RAW264.7 cells.
GEN-27 exhibited anti-inflammatory effects in lipopolysaccharide (LPS)-stimulated RAW264.7 cells. (A) Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay in RAW264.7 cells treated with GEN-27 (1, 5, 10, or 20 μM) for 1 h, followed by 1 μg/mL LPS stimulation for 12 or 24 h. (B) Nitrite level was evaluated by Griess assay after RAW264.7 cells were treated with GEN-27 (1, 5, or 10 μM) for 1 h prior to stimulation with 1 μg/mL LPS for 24 h. (C) Intracellular nitric oxide (NO) release was measured by DAF fluorescence in RAW264.7 cells treated with GEN-27 (1, 5, or 10 μM) for 1 h prior to stimulation with 1 μg/mL LPS for 24 h. *P < 0.05.
GEN-27 suppressed the release of LPS-induced pro-inflammatory cytokines in RAW264.7 cells
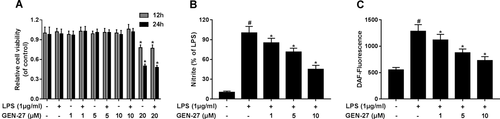
Next, we further analyzed the effects of GEN-27 on LPS-induced pro-inflammatory cytokines such as IL-6, TNF-α, IL-1β, and IL-18. Consistently with the above results, ELISA results manifested that LPS stimulation distinctly enhanced the secreted levels of IL-6 (Figure 2A), TNF-α (Figure 2B), IL-1β (Figure 2C), and IL-18 (Figure 2D) in the cell supernatant of RAW264.7 cells. However, GEN-27 treatment remarkably reversed LPS-induced increase of pro-inflammatory cytokine levels in RAW264.7 cells in a dose-dependent manner.
GEN-27 suppressed the release of lipopolysaccharide (LPS)-induced pro-inflammatory cytokines in RAW264.7 cells. Enzyme-linked immunosorbent assay (ELISA) was conducted to detect the levels of pro-inflammatory cytokines including interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), IL-1β, and IL-18 in RAW264.7 cells exposed to GEN-27 (1, 5, or 10 μM) for 1 h prior to stimulation with 1 μg/mL LPS for 24 h. *P < 0.05.
GEN-27 suppressed LPS-induced iNOS and COX-2 expressions in RAW264.7 cells
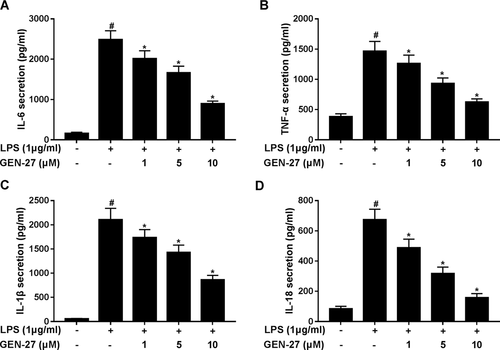
The iNOS and COX-2 are well-known to play major roles in the biosynthesis of NO (Lee et al., 2015). Therefore, we explored the effect of GEN-27 on the expressions of iNOS and COX-2. Western blot and qRT-PCR analysis demonstrated that LPS significantly increased the protein and messenger RNA levels of iNOS and COX-2 in RAW264.7 cells, while the efficacy was attenuated by addition of GEN-27 (Figures 3A and 3C). To clarify the effect of GEN-27 on the transcriptional activation of iNOS gene, luciferase reporter assay was performed in RAW264.7 cells. Results showed that GEN-27 notably blocked the promoter activity of iNOS induced by LPS in a concentration-dependent manner (Figure 3D). Collectively, these results revealed that GEN-27 suppressed the expressions of iNOS and COX-2 in LPS-induced RAW264.7 cells.
GEN-27 suppressed lipopolysaccharide (LPS)-induced inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) expressions in RAW264.7 cells. (A and B) Western blot was performed to detect the protein levels of iNOS and COX-2 in RAW264.7 cells after treatment with GEN-27 (1, 5, or 10 μM) for 1 h, followed by stimulation with 1 μg/mL LPS for 24 h. (C) Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to evaluate the messenger RNA (mRNA) expressions of iNOS and COX-2 in RAW264.7 cells after treatment with GEN-27 (1, 5, or 10 μM) for 1 h, followed by stimulation with 1 μg/mL LPS for 24 h. (D) Luciferase reporter assay was performed in RAW264.7 cells cotransfected with pGL3 empty plasmids or iNOS-Luc and pRL-TK plasmid after treatment with GEN-27 (1, 5, or 10 μM) for 1 h, followed by stimulation with 1 μg/mL LPS for 24 h. *P < 0.05.
GEN-27 inhibited LPS-induced activation of NLRP3 inflammasome in RAW264.7 cells
NLRP3 inflammasome, as one of the most well-characterized inflammasomes, is a multiprotein complex that orchestrates an innate immune response to infection and mediates activation of inflammatory caspases (Elliott and Sutterwala, 2015). It is known that the formation of NLRP3 inflammasome requires the oligomerization of NLRP3, ASC, and caspase 1, which was subsequent production of cytokine IL-1β. Thus, we investigated the effects of GEN-27 on the NLRP3 inflammasome in LPS-stimulated RAW264.7 cells. It has been proposed that ROS is essential for NLRP3 inflammasome activation and subsequent production of cytokine IL-1β (Biasi et al., 2013). We found that LPS stimulation obviously increased ROS production in RAW264.7 cells compared with the control group, which was concentration-dependently retarded following GEN-27 treatment (Figure 4A). Moreover, GEN-27 drastically repressed LPS-induced activation of caspase 1 activity in RAW264.7 cells in a dose-dependent manner (Figure 4B). Western blot analysis further implicated that the protein levels of NLRP3, ASC, and caspase 1 were strikingly augmented in LPS-treated RAW264.7 cells, while these changes were substantially suppressed after GEN-24 administration (Figures 4C and 4D). To further validate this effect, RAW264.7 cells were exposed to GEN-27 (1, 5, or 10 μM) for 1 h before treatment with 5 mM ATP for 24 h, which has been recognized as an NLRP3 inflammasome activator (Ozaki et al., 2015). The results demonstrated that the protein levels of NLRP3 and the subsequent release of IL-1β were conspicuously improved by ATP treatment in RAW264.7 cells, which was prominently suppressed by cotreatment with GEN-27 and ATP in a dose-dependent manner (Figures 4E and 4F). These results suggested that GEN-27 inhibited LPS-induced activation of NLRP3 inflammasome in RAW264.7 cells.
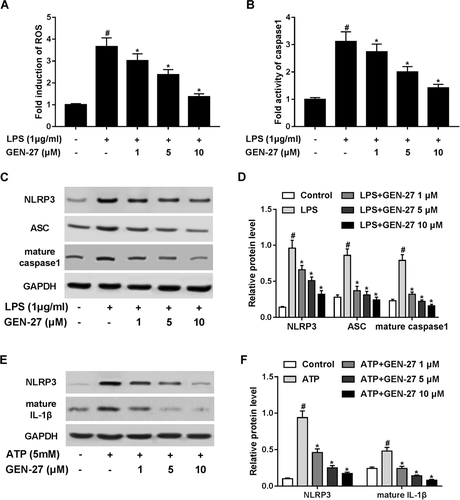
GEN-27 inhibited lipopolysaccharide (LPS)-induced activation of NOD-like receptor 3 (NLRP3) inflammasome in RAW264.7 cells. (A) Intracellular reactive oxygen species (ROS) production was measured by DCF fluorescence in RAW264.7 cells treated with GEN-27 (1, 5, or 10 μM) for 1 h, followed by stimulation with 1 μg/mL LPS for 24 h. (B) Caspase 1 activity was assessed by a commercial assay kit in RAW264.7 cells treated with GEN-27 (1, 5, or 10 μM) for 1 h, followed by stimulation with 1 μg/mL LPS for 24 h. (C and D) Western blot was applied to determine the protein levels of NLRP3, apoptosis-associated speck-like protein-containing a CRAD (ASC), and caspase 1 in RAW264.7 cells treated with GEN-27 (1, 5, or 10 μM) for 1 h before stimulation with 1 μg/mL LPS for 24 h. (E and F) The protein levels of NLRP3 and IL-1β were determined by western blot in RAW264.7 cells treated with GEN-27 (1, 5, or 10 μM) for 1 h before stimulation with 5 mM adenosine triphosphate (ATP) for 24 h. *P < 0.05.
GEN-27 inhibited LPS-induced NF-κB pathway activation in RAW264.7 cells
It is well-known that activation of NF-κB pathway and nuclear translocation of NF-κB p65 play a crucial role in controlling inflammatory mediator expression and mediating the inflammatory response (Yenari and Han, 2006). To further analyze the anti-inflammatory mechanism of GEN-27, western blot was performed to measure the expressions of characteristic proteins in NF-κB pathway including p65 and p-p65. The results showed that LPS stimulation significantly enhanced the ratio of p-p65/p65 in RAW264.7 cells, while GEN-27 treatment strikingly attenuated LPS-induced increase of p-p65/p65 ratio (Figures 5A and 5B). Moreover, GEN-27 also concentration-dependently suppressed the increase of transcriptional activity of NF-κB induced by LPS in RAW264.7 cells (Figure 5C). Collectively, we concluded that GEN-27 remarkably impeded LPS-induced activation of NF-κB pathway in RAW264.7 cells.
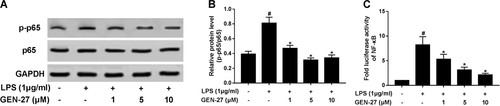
GEN-27 inhibited lipopolysaccharide (LPS)-induced nuclear factor (NF-κB) activation in RAW264.7 cells. (A and B) Western blot was performed to determine the protein levels of p-p65 and p65 in RAW264.7 cells treated with GEN-27 (1, 5, or 10 μM) for 1 h, followed by stimulation with 1 μg/mL LPS for 24 h. (C) RAW264.7 cells were cotransfected with NF-κB-Luc and pRL-TK plasmid. After 24 h of transfection, the cells were treated with GEN-27 (1, 5, 10 μM) for 1 h, followed by stimulation with 1 μg/mL LPS for a further 24 h, followed by determination of luciferase activity by luciferase reporter assay.
Discussion
Inflammation is a complex pathophysiological phenomenon that protects the host against numerous pathogens. It has been proposed that the excessive production or release of the pro-inflammatory cytokines in response to stimuli in macrophages is a major contributing factor in the initiation and deterioration of many inflammatory diseases (Son et al., 2014). Hence, blockage of the release of inflammatory mediators may be a promising therapeutic strategy to treat inflammatory disorders. Herein, we showed that GEN-27 exerted anti-inflammatory roles in LPS-stimulated RAW264.7 cells by inhibiting the release of inflammatory mediators. This effect was mediated by suppressing the activation of NLRP3 inflammasome and NF-κB pathway. To our knowledge, our study is the first evidence demonstrating the anti-inflammatory effects of GEN-27 on LPS-induced inflammation and revealing its underlying mechanism.
Macrophages play a crucial role in the progress of inflammation by releasing excessive pro-inflammatory mediators, which ultimately cause clinical symptoms (Kobayashi, 2010). LPS, a potent endotoxin of Gram-negative bacteria, stimulates inflammatory responses by activating macrophages (Gioannini and Weiss, 2007). GEN, one of the major bioactive components of soybeans, exerts anti-inflammatory activity. However, whether GEN-27, a GEN derivative, exerts anti-inflammatory effects on LPS-induced inflammation in macrophages remains largely unknown. Therefore, we investigated the effects of GEN-27 on LPS-induced inflammatory mediator expression and unravel the exact signaling pathway in RAW264.7 cells. In our study, we found that GEN-27 administration dose-dependently inhibited nitrite and NO levels in LPS-stimulated RAW264.7 cells. Moreover, GEN-27 treatment ablated the release of LPS-induced pro-inflammatory cytokines and expressions of iNOS and COX-2 in RAW264.7 cells. During the process of inflammation, excessive NO is produced after the activation of iNOS in the LPS-induced inflammatory response of macrophages (Cao et al., 2012). COX-2 is also involved in NO production under inflammatory conditions. Thus, these results suggested that GEN-27 exerted anti-inflammatory effects via suppression of the expressions of iNOS and COX-2.
It is well-known, NLRP3 inflammasome, a cytosolic multiprotein complex, is involved in the development and progression of many inflammatory disorders (Coll et al., 2015). Once activated by stimuli, such as LPS, NLRP3 recruits the adaptor protein ASC and in turn promotes the recruitment and activation of procaspase-1, resulting in the production and maturation of IL-1β (Abderrazak et al., 2015). In addition, it has been proposed that ROS derived from damaged mitochondria plays an essential role in the activation of NLRP3 inflammasome by stimuli such as LPS (Martinon, 2010). In our study, we found that GEN-27 attenuated LPS-induced ROS production, as well as mitigated the increase of caspase 1 activity and the protein levels of NLRP3, ASC, and caspase 1 in LPS-treated RAW264.7 cells. Moreover, GEN-27 also dose-dependently offset the elevation of NLRP3 and IL-1β levels triggered by ATP, a NLRP3 inflammasome activator, in RAW264.7 cells. These results indicated that GEN-27 inhibited LPS-induced NLRP3 inflammasome activation in RAW264.7 cells.
In addition to NLRP3 inflammasome, the inflammatory process is also tightly controlled by transcription factor NF-κB, which is responsible for the expressions of iNOS, COX-2, and various inflammatory cytokines including TNF-α, IL-1β, and IL-6 (Lee et al., 2012). NF-κB is localized in the cytoplasm in an inactive form and translocate into the nucleus as an active transcription factor to initiate the inflammatory response (Gilmore, 2006). Also, the aberrant activation of NF-κB is reported to contribute to the pathogenesis of inflammatory diseases (Liu and Malik, 2006). Meanwhile, it has also been convincingly demonstrated that NF-κB signaling pathway plays an essential role in the activation of NLRP3 inflammasome (Lamkanfi and Dixit, 2014). In our study, we found that GEN-27 treatment significantly suppressed LPS-induced phosphorylation of NF-κB p65, which, in turn, concentration-dependently suppressed LPS-induced increase of transcriptional activity of NF-κB in RAW264.7 cells. According to these above results, we concluded that GEN-27 exerted anti-inflammatory effects by suppressing the activation of NLRP3 inflammasome and NF-κB pathway. The available evidence has indicated a potential mechanism that GEN-27 suppressed LPS-induced activation of NF-κB p65 by blocking the phosphorylation of IKBα and IKKα/β in colorectal cancer cells (Wang et al., 2016). Hence, we hypothesized it might be one of the main mechanisms underlying GEN-27 regulating NF-κB p65 in RAW264.7 cells and it is expected to be elucidated in the future.
In summary, our study demonstrated the protective effects of GEN-27 on LPS-induced inflammation in RAW264.7 cells. GEN-27 exerted anti-inflammatory effects in LPS-stimulated RAW264.7 cells by suppressing the activation of NLRP3 inflammasome and NF-κB pathway, suggesting that GEN-27 may be a promising therapeutic agent for the prevention and therapy of inflammatory-associated diseases.
Conflict of interests
The authors declare that there are no conflict of interests.




