Mesencephalic astrocyte-derived neurotrophic factor reduces cell apoptosis via upregulating GRP78 in SH-SY5Y cells
Abstract
Mesencephalic astrocyte-derived neurotrophic factor (MANF) protects dopaminergic neurons from damage. In this study, we used MTT, immunohistochemistry, and TUNEL staining to investigate the protective effect of MANF in SH-SY5Y cells treated with 6-OHDA or overexpressed α-synuclein. Cleaved caspase-3 levels significantly increased in cells treated with 6-OHDA or overexpressed α-synuclein. 6-OHDA or α-synuclein overexpression that induced cleaved caspase-3 levels to increase was reduced by MANF treatment. In addition, MANF treatment upregulated GRP78 expressions in cells treated with 6-OHDA or overexpressed α-synuclein, and RNAi knockdown for GRP78 could block the MANF induced cell survival from 6-OHDA treatment. Furthermore, GRP78 overexpression inhibited 6-OHDA-induced apoptosis. Our data suggest that MANF inhibits apoptosis induced by 6-OHDA and overexpressed α-synuclein in SH-SY5Y cells via upregulating GRP78 in the transcriptional pattern.
Abbreviations
-
- 6-OHDA
-
- 6-hydroxydopamine
-
- BDNF
-
- brain-derived neurotrophic factor
-
- ER
-
- endoplasmic reticulum
-
- GDNF
-
- glial cell line-derived neurotrophic factor
-
- GRP78
-
- glucose-regulated protein 78
-
- MANF
-
- mesencephalic astrocyte-derived neurotrophic factor
-
- PD
-
- Parkinson's disease
Introduction
Parkinson's disease (PD) is characterized by progressive degeneration and loss of dopaminergic neurons in the substantia nigra. The pathogenesis of PD is related to several factors such as hereditary and environmental factors and oxidative stress (Apostolou et al., 2008). Although several neurotrophic factors, such as glial cell line-derived neurotrophic factor (GDNF) or brain-derived neurotrophic factor (BDNF), have been applied to protect dopaminergic neurons, clinical trials have shown that these neurotrophic factors exhibit low efficacy in treating PD patients (Barker, 2006). A novel mesencephalic astrocyte-derived neurotrophic factor (MANF) isolated from astrocytes exhibits a survival-promoting effect to dopaminergic neurons in vitro and in 6-hydroxydopamine (6-OHDA)-treated PD rats (Dorner et al., 1992; Cooper et al., 2006; Gorbatyuk et al., 2012; Bobela et al., 2014). However, the neuroprotective mechanisms of MANF in dopaminergic neurons remain unclear.
Endoplasmic reticulum (ER) stress plays an important role in the development of PD. The most studied ER stress-related protein in PD is α-synuclein, which is the main component of Lewy bodies and an intranuclear inclusion body (Grondin et al., 2002; Hara et al., 2011). Overexpressed or mutated α-synuclein, such as A53T and A30P, induces oxidative stress and ER stress. Under chronic ER stress, dopaminergic neurons undergo apoptosis that eventually lead to PD. It has been reported that MANF improves the survival of dopaminergic neurons induced by ER stress (Imaizumi et al., 2001; Holtz and O'Malley, 2003). However, to date, it remains unclear whether MANF inhibits α-synuclein-induced neurotoxicity in dopaminergic neurons.
Signaling mechanisms by which MANF protects dopaminergic neurons from ER stress remain unclear. GRP78, which is located in the lumen of the ER, is upregulated by ER stress and plays a key role in inhibiting ER stress. It has been reported that GRP78 overexpression inhibits apoptosis induced by oxidative stress and ER stress (Kirik et al., 2002; Lee, 2005). We hypothesize that MANF exhibits its neuroprotection via upregulation of GRP78.
In this study, we investigated the effect of MANF on apoptosis in dopaminergic SH-SY5Y cells treated with 6-OHDA or overexpressed α-synuclein and examined GRP78 expressions in MANF-treated cells. This study aims to determine whether MANF protected dopaminergic neurons from apoptosis via upregulation of GRP78.
Materials and methods
MANF purification
MANF protein was purified as previously reported. Briefly, bacteria expressing MANF cDNA were cultured in LB medium overnight. Then, bacteria were collected by centrifugation and lysed using a sonicator. Supernatants were collected, and MANF protein with a poly-HIS tag was purified by Co2+ affinity column and eluted with 40 mM of imidazole, followed by elution with PBS using an ultrafiltration column. MANF protein concentration was examined by a BCA quantification kit, and purity was verified using SDS–PAGE.
Cell culture
SH-SY5Y cells were cultured in a T75 flask with Dulbecco's modified Eagle's medium containing nutrient mixture F-12 with 10% fetal bovine serum. Cells were harvested by trypsin-EDTA solution and subcultured in six-well plates at a density of 106 cells/well 1 day before the experiment. Cells were treated with 50, 60, or 100 µM of 6-OHDA for 48 h, and were used for the following experiments.
Cell transfection
Cells were seeded in six-well plates at a density of 106 cells/well and cultured for 1 day. Then, cells were transfected with plasmids expressing α-synuclein and GRP78 using a Lipofectamine® 3000 Reagent (L3000-008; Invitrogen, USA), according to manufacturer's protocols. Cells were cultured in an incubator at 37°C with 5% CO2 for 48 h.
MTT
After the cells had been incubated with 6-OHDA and MANF in 96-well plate as prescribed in the experiments, 10 µL of stock-solution of MTT was added to each well, and after 4 h of incubation at 37 °C, 100 µL of solubilization solution was added to each well. The ODs at 490 nm were measured using a Bio-Rad iMark 96-well scanner subsequent to incubation at 37°C until formanzan was totally disolved.
RNAi transfection in SH-SY5Y cells
For GRP78 RNAi knockdown experiment in SH-SY5Y cells, we design the target site of small hairpin RNAs (shRNA) for GRP78 from 3,476 to 3,494 cDNA (NM_005347). Synthesized shRNA template oligonucleotides were phosphorylated, annealed, and then ligated into linearized PLV-shRNA clotech vector digested with EcoRI/BamHI. SH-SY5Y were transfected with shRNA plasmid by Lipofectamine 3000 reagent (Invitrogen) and then were treated with drugs.
Western blot analysis
Cells were homogenized in RIPA lysis buffer. Lysates were centrifuged at 14,000 rpm for 3 min, and protein concentrations in the supernatant were analyzed using a BCA Protein Quantitation Kit. Proteins (30 µg) were loaded to each lane and separated by electrophoresis on SDS–PAGE gels, followed by transferring to PVDF membranes. Membranes were incubated with 5% BSA for 1 h. Then, membranes were incubated with primary antibodies against caspase-3 (9579; Cell Signaling Technology, USA), α-synuclein (2642; Cell Signaling Technology), and GRP78 (3183; Cell Signaling Technology), as well as MANF (H00007873-M01; Abnova, USA), overnight at 4°C. Membranes were incubated with horseradish-linked secondary antibodies (HA1001-100; Huabio, Hangzhou, China) for 2 h at 37°C. Bands were visualized using an ECL detection system.
TUNEL staining
TUNEL staining was performed according to manufacturer's protocols. Briefly, cells were washed with PBS three times and fixed in 4% paraformaldehyde in PBS. Cells were treated with 50 µL of TUNEL reaction mixture for 1 h at 37°C, then washed three times with PBS. Cells were counterstained with DAPI for 5 min and examined under a fluorescence microscope.
RNA isolation, reverse transcription, and quantitative PCR
RNA from culture cells was isolated by SV Total RNA Isolation System (Promega Corporation) according to the manufacturer's instruction. Reverse transcription reactions were performed with PrimeScriptTM RT Master Mix (Takara Bio, Inc.) for 40 min in 37°C according to the manufacturer's protocol. Quantitative PCR was performed using iTaqTM Universal SYBR® Green Supermix (Bio-Rad Laboratories, Inc.) and ABI7500 Real-Time PCR System. The expression levels were normalized to the levels of β-actin in the same samples.
Statistical analysis
Analyses were performed using SPSS 13.0. One-way ANOVA was used to compare differences among groups, followed by Tukey's test. A P-value <0.05 was considered significant.
Results
Purification and functional identification of MANF
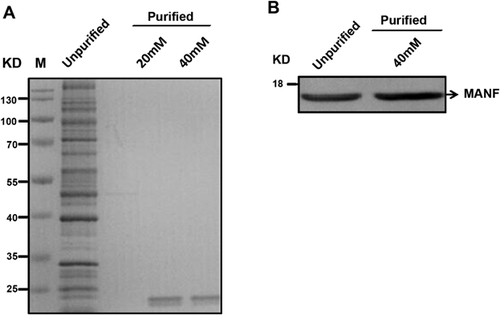
MANF proteins were purified through Ni-chelating affinity chromatography and identified as an 18-kD band, as detected by SDS–PAGE and Western blot after elution with 40 mM of imidazole (Figures 1A and 1B).
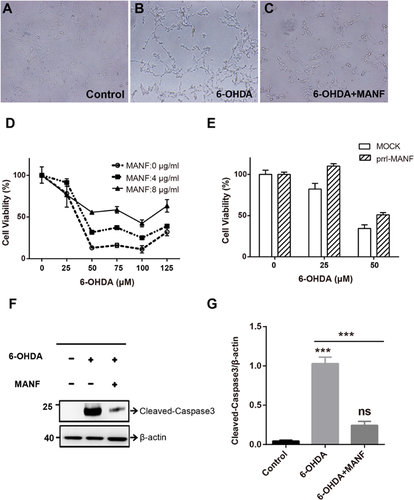
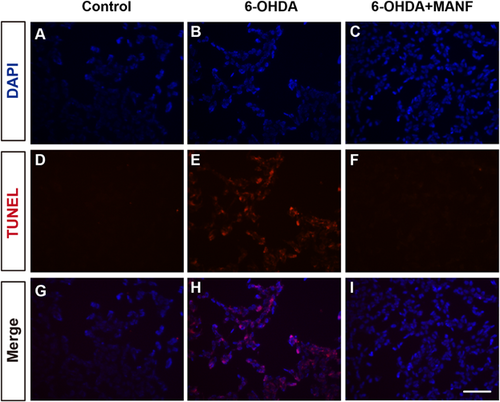
To identify the function of purified MANF, we examined the protective effect of MANF on 6-OHDA-induced injury in SH-SY5Y cells. 6-OHDA treatment induces morphological damage in SH-SY5Y cells. The addition of MANF prevented 6-OHDA-induced cell damage (Figures 2A–2C). With MTT method, we found that either exogenous MANF treatment or endogenous MANF over expression could improve the viabilty of SH-SY5Y, which was damaged by 6-OHDA (Figures 2D and 2E). 6-OHDA significantly increased cleaved caspase-3 expressions in SH-SY5Y cells, and MANF treatment significantly inhibited the increase in cleaved caspase-3 expressions induced by 6-OHDA (Figures 2F and 2G). These results suggest that purified MANF protein protected cells from 6-OHDA-induced apoptosis. Furthermore, TUNEL staining revealed that MANF reduced 6-OHDA-induced apoptosis in SH-SY5Y cells, which was consistent with Western blot results (Figure 3).
MANF inhibits α-synuclein-induced apoptosis in SH-SY5Y cells
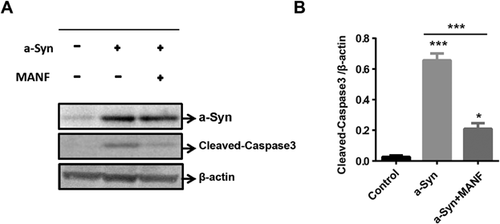
Cleaved caspase-3 expressions significantly increased in SH-SY5Y cells overexpressed with α-synuclein, compared with control cells (Figures 4A and 4B). Forty-eight hours of MANF (4 µg) treatment significantly inhibited the increase in cleaved caspase-3 expression induced by overexpressed α-synuclein (Figures 4A and 4B). These results suggest that MANF inhibited α-synuclein-induced cell apoptosis.
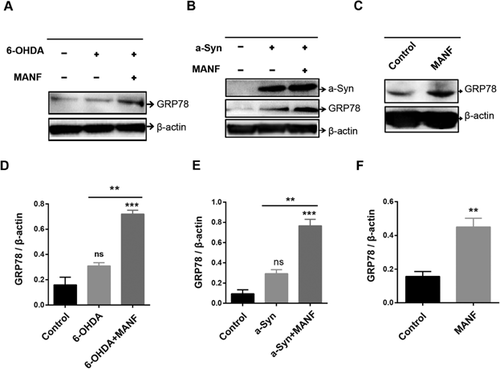
MANF upregulates GRP78 expressions in SH-SY5Y cells
We investigated the effect of MANF on GRP78 expressions in SH-SY5Y cells treated with 6-OHDA. GFP78 expression levels did not significantly increase in cells treated with 6-OHDA compared with control cells. MANF treatment resulted in a significant increase in GRP78 expression in 6-OHDA-treated cells (P < 0.05; Figures 5A and 5D). In addition, GRP78 expressions significantly increased in α-synuclein overexpressed cells than in control cells (Figures 5B and 5E). MANF treatment significantly upregulated GRP78 expressions in α-synuclein overexpressed cells (P < 0.05; Figures 5B and 5E). To validate the effect of MANF in upregulating the expression of GRP78, we tested the effect of MANF on the expression of GRP78 in control cells. Results revealed that MANF treatment significantly increased GRP78 expression in control cells (Figures 5C and 5F), suggesting that MANF upregulated the expression level of GRP78 in SH-SY5Y cells.
MANF inhibits 6-OHDA-induced apoptosis in SH-SY5Y cells through transcriptional increase of GRP78 level
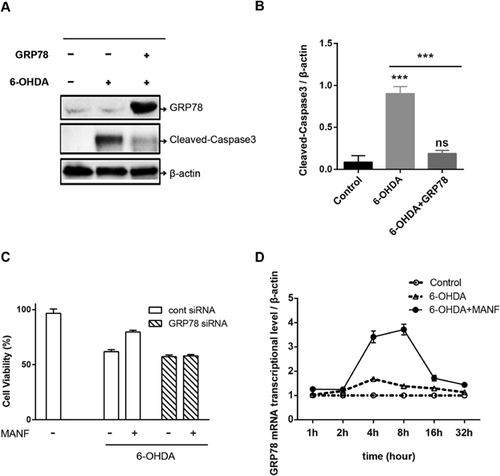
Several lines of evidence have shown that GRP78 inhibited apoptosis induced by α-synuclein overexpression (Lew, 2007). However, there is no direct evidence showing that GRP78 protects cells from 6-OHDA-induced cell apoptosis. GRP78 overexpression significantly inhibited the increase in cleaved caspase-3 expression induced by 6-OHDA in SH-SY5Y cells (Figures 6A and 6B). Furthermore, we showed that RNAi knockdown for GPR78 could block MANF-induced survival of SH-SY5Y (Figure 6C). These results suggest that GRP78 inhibited 6-OHDA-induced apoptosis in SH-SY5Y cells. To determine whether the increase of GRP78 induced by MANF is transcriptional or posttrascriptional, we performed the RT-PCR of GRP78 in 6-OHDA-treated SH-SY5Y. The result showed that the relative mRNA level of GRP78/β-actin was elevated at 2–16 h followed the MANF treatment (Figure 6D). These indicated that MANF inhibits 6-OHDA-induced apoptosis in SH-SY5Y cells through transcriptional increase of GRP78 level.
Discussion
MANF inhibits α-synuclein-induced apoptosis
Recently, it has been reported that MANF is widely distributed in various neurons and cells outside the nervous system (Lindholm and Saarma, 2010) and that MANF has been recognized as an important factor that plays an important neuroprotective role in a rat model of PD (Cooper et al., 2006). MANF has been reported to protect dopaminergic neurons in the substantia nigra from injury induced by multiple toxins (Cooper et al., 2006; Lindholm et al., 2008). Similarly, we found that MANF inhibited apoptosis in SH-SY5Y cells treated with 6-OHDA or overexpressed α-synuclein.
In this study, we found that MANF suppressed 6-OHDA-induced apoptosis in SH-SY5Y cells. 6-OHDA is a neurotoxin widely used to create animal models of PD. We found that 6-OHDA increased the activated caspase-3, a marker of apoptosis. MANF treatment inhibited the increasing of cleaved caspase-3 induced by 6-OHDA. Our results suggest that MANF plays an important role in suppressing cell apoptosis induced by 6-OHDA. Our findings are consistent with recent studies, showing that MANF inhibits cell apoptosis (Cooper et al., 2006).
Mainly located presynaptically, α-synuclein is an indispensable protein in the development of the nervous system. Abnormal accumulation of α-synuclein has been found in PD brains, and α-synuclein overexpression induces injury to dopamine neurons (Liu et al., 1997). To the best of our knowledge, we found for the first time that MANF reversed the apoptosis of α-synuclein overexpressed cells in this present study.
MANF alleviates ER stress through GRP78 upregulation
ER stress contributes to the pathogenesis of PD (Nuss et al., 2008). The activation of ER stress is often companied by GRP78 upregulation (Petrova et al., 2003; Palgi et al., 2009), which has been found to protect cells from degradation induced by ER dysfunction (Kirik et al., 2002; Lee, 2005). Previous studies have shown that MANF reduces ER stress and inhibits ER stress-induced neuronal apoptosis (Holtz and O'Malley, 2003; Serres and Carney, 2006). In this present study, we investigated the effect of MANF on the expression of GRP78 in SH-SY5Y cells treated with 6-OHDA or overexpressed α-synuclein, which are two cell models of PD. We found that MANF treatment significantly upregulated GRP78 levels in both PD cell models, which was accompanied by a decrease in cleaved caspase-3 expression, suggesting that MANF alleviates ER-induced apoptosis through GRP78 upregulation.
Although GRP78 overexpression reduced neurotoxicity induced by the abnormal accumulation of α-synuclein in a rat model of PD (Lew, 2007), it remains unknown whether GRP78 overexpression can rescue 6-OHDA-induced cell injury. In this present study, we found that GRP78 overexpression inhibited the increase in active caspase-3 levels induced by 6-OHDA, suggesting that GRP78 upregulation may protect cells from 6-OHDA-induced apoptosis. Interestingly, GRP78 has been demonstrated as a neuroprotective factor to slow the progression of a variety of neurodegenerative diseases (Shen et al., 2002; Lew, 2007). For example, in vivo and in vitro studies have shown that GRP78 levels in ER is robustly reduced in neurons treated with neurotoxin MPTP/MPP+, leading to cell apoptosis (Shen et al., 2002; Lew, 2007). In addition, the amount of GRP78 gradually reduces with age (Smith et al., 2005). The amount of GRP78 is smaller in livers of older mice than in younger mice by approximately 20% (Smith et al., 2005). This may explain why older mice are more susceptible to age-related diseases such as Alzheimer's disease and PD (Shen et al., 2002; Nuss et al., 2008). Several neurodegenerative diseases including PD are age-related (Tadimalla et al., 2008) and, thus, these may be associated with the reduced amount of GRP78. Our findings, which revealed that MANF upregulated GRP78 in SH-SY5Y cells with a decrease in cell apoptosis, suggest that MANF may protect neurons from cell apoptosis via upregulation of GRP78, thus, leading to the inhibition of the development of PD.
Although several factors that could lead to apoptosis also can upregulate GRP78 via ER stress, such as 6-OHDA and a-synuclein, there is few protective effect. It might because the influence of upregulated GRP78 is too small to inhibit apoptosis. A similar result that thapsigargin, an inducer of GRP78, could provide protection against 6-hydroxydopamine-induced cytotoxicity was proved the mass of upregulation of GRP78 could have protective effects for cytotoxin (Voutilainen et al., 2009). Besides, in some cases, Bip/Grp78 expression level is lower in MANF-treated group than in vehicle but injured group (Yang et al., 2014). This phenomenon may be explained by compensatory mechanism. Bip/Grp78, belonging to HSP family, is involved in survival signal transduction and is liable to be upregulated under the environment of cell stress. While cell stress is eliminated by MANF treatment, Bip/Grp78 would be reduced in feedback loop.
In conclusion, we found that MANF inhibited neuronal apoptosis induced by neurotoxin 6-OHDA and overexpressed α-synuclein through GRP78 upregulation. Our study provides a new theoretical basis for using neurotrophic factors in treating PD.
Acknowledgment and funding
This study is supported by the National Major Scientific and Technological Special Project for Significant New Drugs Development (2014ZX09102043-003), National Natural Science Foundation of China (81371403), Shanghai Science and Technology Commission (13JC1401102), Natural Science Foundation of Jiangsu Province of China (BK20140275), and Suzhou science and technology projects (ZXY2012029).
Conflict of interest
The authors have declared that no conflicts of interest exist.
Authors' contribution
All authors had full access to all the data in the study and take responsibility for the integrity and the accuracy of the data analysis. Study concept and design: Jingwei Huang, Jianmin Fang, Lingjing Jin. Acquisition of data: Jingwei Huang, Changyan Chen, Hua Gu, Chen Li. Analysis and interpretation of data: Jingwei Huang, Changyan Chen, Xing Fu, Ming Jiang, Hui Sun, Jun Xu. Drafting of the manuscript: Jingwei Huang, Jianmin Fang, Lingjing Jin. Critical revision of the manuscript for important intellectual content: Hua Gu, Chen Li, Xing Fu, Ming Jiang, Hui Sun, Jun Xu. Statistical analysis: Changyan Chen, Hua Gu, Chen Li, Xing Fu, Ming Jiang, Hui Sun, Jun Xu. Obtained funding: Jianmin Fang, Lingjing Jin.




