A combined approach against tumorigenesis using glucose deprivation and mitochondrial complex 1 inhibition by rotenone
Abstract
Cancer cells exhibit various degrees of mitochondrial metabolic alterations. Owing to their multiple roles, mitochondria are attractive target for cancer therapy. Cancerous cells have high glucose (HG) requirements for their growth. Depriving them of glucose has been an approach used in many studies to restrict their perpetuation. However, such deprivation can negatively affect the surrounding normal cells in vivo. Keeping this in view, we treated HeLa cells with only physiological glucose (PG, 5.5 mM) and a combination of physiological glucose with a very low dose (1 nM) of rotenone (PGT), taking high glucose (HG, 25 mM)-treated HeLa cells as normal. We demonstrated that HeLa cells under PG condition mainly exhibited growth arrest. The PGT combination induced apoptosis in HeLa cells by generation of ROS, decrease in ATP production even with around 1.89-fold increase in glucose consumption, cell cycle arrest at S-phase and substantial increase in sub-diploid (Sub-D) population. The oxidative stress generated in both PG and PGT conditions stabilised p53 by localising it in the nuclei of HeLa cells, which would have otherwise undergone HPV-mediated inactivation. Pre-mature senescence induced due to limited glucose availability was found to be regulated by nuclear translocated p53 which, in turn, induced p21, pAkt and pERK. The cyto-toxic effect of rotenone on glucose deprived HeLa cells, synergistically activated NFκB, caspase-3 and Bax along with reduced expression of hyaluronan, a ROS scavenging molecule on their cell surface. Thus, our finding might be a valuable approach to specifically target cancerous cells in a more physiologically feasible condition and can serve as a relevant biochemical basis to gain new insights into cancer therapy.
Abbreviations
-
- AF
-
- auto-fluorescence
-
- AP
-
- alkaline phosphatase
-
- ATP
-
- adenosine 5′-triphosphate
-
- BCIP
-
- 5-bromo-4-chloroindol-3-yl phosphate
-
- BSA
-
- bovine serum albumin
-
- DAPI
-
- 4′,6-diamidino-2-phenylindole
-
- DMEM
-
- Dulbecco's modified Eagle's media
-
- DMSO
-
- dimethyl sulfoxide
-
- ECM
-
- extra cellular matrix
-
- ETC
-
- electron transport chain
-
- FACS
-
- fluorescence-activated cell sorting
-
- FBS
-
- fetal bovine serum
-
- FSC-A
-
- forward scatter-area
-
- HA
-
- hyaluronan
-
- H2DCFDA
-
- 5,6-chloromethyl-29,79-dichlorodihydro-fluoresceindiacetate
-
- HG
-
- high glucose
-
- HPV
-
- human papilloma virus
-
- MTT
-
- 3-(4,5-dimethyl-thiazol-2-Yl-2,5-diphenyltetrazolium bromide)
-
- NBT
-
- nitro blue tetrazolium
-
- PBS
-
- phosphate buffer saline
-
- PG
-
- physiological glucose
-
- PGT
-
- physiological glucose with 1 nm rotenone treatment
-
- PI
-
- propidium iodide
-
- PVDF
-
- poly-vinylidine di fluoride
-
- RIPA Buffer
-
- radio immuno-precipitation assay buffer
-
- ROS
-
- reactive oxygen species
-
- RT
-
- room temperature
-
- SA-β-gal
-
- senescence-associated β-galactosidase
-
- SDS–PAGE
-
- sodium dodecyl sulphate–polyacrylamide gel electrophoresis
-
- Sub-D
-
- sub-diploid population
Introduction
Cancer cells have sweet tooth and have much higher rate of glucose consumption. Uncontrolled proliferation of tumour cells using nutrients from their environment is stimulated by growth factors acquired through genetic mutations which alters receptor initiated signalling pathways. According to Warburg (1956), cancer cells adopt anaerobic metabolism as a means of survival after injury to its respiratory system. So, tumours are initiated by persistent damage to the mitochondrial number, shape and function (Pedersen, 1978). Despite the damage, mitochondria play important role in maintaining mitochondrial potential and oxidative equilibrium which are essential for cell viability and apoptotic control. Many natural and synthetic compounds which affect mitochondria are reported to show anticancer action (Chen et al., 2010). Rotenone, a plant compound derived from the Leguminosae family, which directly targets mitochondrial NADH: ubiquinone oxido-reductase, that is, complex I of electron transport chain (ETC pathway), induces cell death through the generation of reactive oxygen species (ROS) in mitochondria that can readily influence mitochondrial function without having to cope with long diffusion times from cytosol (Armstrong et al., 2001). As it may (Armstrong et al., 2001) or may not (Klintworth et al., 2007) induce cell death, the mechanism underlying rotenone-induced apoptosis in different cancer cells is not completely understood and whether its action can be altered by glucose deprivation is also not clear yet.
For peculiar metabolism, cancer cells are particularly sensitive to treatments inhibiting glycolysis and glucose deprivation (Pelicano et al., 2006), as in both circumstances they lose hyper-proliferative ability and ultimately die (ElMjiyad et al., 2011). Assuming interference of high glucose in drug response to cancer cells, here we tried to investigate low-dose rotenone-induced (1 nM) toxicity on HeLa cells, the human cervical carcinoma cell line, under normoglycemic (5.5 mM) condition against hyperglycaemia (25 mM), reported to be normal growth requirement for cancer cells. Under glucose depletion, HeLa cells are forced to utilise mitochondria for energy generation through oxidative phosphorylation (Rossignol et al., 2004). As a result of forced dependency on mitochondria and subsequent complex, one blocking by rotenone may provide synergistic inhibitory effect on HeLa cells growth and change in protein signalling cascade. Therefore, present study suggested that combined treatment targeting both glycolysis and mitochondria complex I inhibition, exploiting peculiar features of cancer cells, might efficiently target growth of HeLa cells.
Materials and methods
Chemicals
Low (5.5 mM) and high (25 mM) glucose DMEM and antibiotics for cell culture, some antibodies nuclear stain (DAPI), ATP bioluminescent assay kit and glucose estimation kit were bought from Sigma–Aldrich, USA while the FBS was obtained from Invitrogen (Gibco). Antibodies were obtained from Santa Cruz Biotechnology, Inc., Abcam, Sigma–Aldrich (USA) and Molecular Probes, Inc. All other chemicals used were standard molecular grade.
Cell culture and treatment
HeLa cells were seeded in high glucose DMEM (25 mM glucose) (HG) containing 10% FBS with 50 μg/mL fungizone and 100 μg/mL streptomycin under a humidified atmosphere of 5% CO2 at 37°C. They were sub-cultured at ∼80% growth in 10% FBS HG and maintained further in 10% FBS physiological glucose DMEM (5.5 mM Glucose) (PG). After sub-confluent growth in 10% FBS with PG, the cells were treated with 1 nm concentration of rotenone (PGT) in dark for 3 days (72 h) with treatment renewal each day in fresh medium.
Cell viability assay (MTT assay)
HeLa cells seeded in a 96-well plate (103 cells/well) were treated along with appropriate controls. At required time points, 4 μL/well MTT stock (5 mg/mL) was added and incubated in dark at 37°C for 2 h. Two hundred microlitre of DMSO was added in each well to solubilise the formazan crystals. Absorbance was measured at 570 nm with a Varian UV-visible Spectrophotometer.
Assay of intracellular reactive oxygen species using H2DCFDA
Cultured HeLa cells grown in 6-well plates were incubated with 10 μM H2DCFDA solution in dark for 30 min and then washed with PBS. Cells from each well were scrapped out in 200 μL of PBS in separate eppendorf tubes. Fluorescence readings were taken at an excitation wavelength of 488 nm and emission wavelength of 530 nm within 10 min.
ATP assay
HeLa cells were assayed for ATP content using Adenosine 5′-tri-phosphate (ATP) Bioluminescent Assay kit (Sigma–Aldrich, USA) as per manufacturer's protocol.
Assay for glucose consumption
The media in which HeLa cells were grown and treated (HG, PG and PGT) in 6-well plates were assayed for glucose consumption after 72 h using glucose assay kit (Sigma–Aldrich, USA) as per manufacturer's protocol. The results were obtained from three independent experiments.
Immunoblot analysis
Cell lysates were prepared using RIPA buffer and then electro-phorosed by SDS–PAGE and then electro-blotted at 150 mA for 3 h on PVDF membrane. After blocking of non-specific sites with 1.5% BSA for 1 h at room temperature (RT), it was incubated with desired primary antibody overnight at 4°C. The PVDF membrane was then washed thrice with PBST (PBS with 0.1% Tween 20) and incubated for 1 h with either AP-conjugated secondary antibody. Blots were developed using NBT (Nitro Blue Tetrazolium) with BCIP (5-bromo-4-chloroindol-3-yl phosphate) colorimetric system.
Immuno-cytochemical analysis and fluorescence microscopy
Cells were grown on cover-slips and then fixed with 2% p-formaldehyde in PBS (pH 7.2) for 15 min followed by permeabilisation with 0.1% Triton X-100 for 1 min. The cells were then washed with PBS (pH 7.2) and blocked with 3% BSA for 1 h at RT. Incubation with primary antibody in 1.5% BSA for 1 h at RT was performed. Cells were washed thrice with PBS and then processed with secondary antibody which were tagged either with Cy3 (Sigma) or Alexa Fluor 546. DAPI was used for nuclear staining. Cells washed thrice with PBS vigorously and mounted in 50% glycerol. Slides were viewed under a Zeiss fluorescence microscope equipped with an epi-fluorescence and Axiocam camera system coupled with axiovision software (CarlZeiss, Germany).
Auto-fluorescence and cell size analysis by FACS
Harvested cells were suspended in 1 mL PBS and analysed immediately to avoid cell aggregation for changes in auto-fluorescence (AF) (450–490 nm) and cell size (FSC-A). The flowcytometric analysis was performed on 20,000 events using BD-FACS Verse System equipped with BD FACS Suite Software for data analysis. The results represent three independent experiments. Cell debris with very low forward/side scatter was excluded from the analysis.
Senescence-associated β-galactosidase activity assay
Cells grown on cover slips were fixed with 2% formaldehyde, washed with PBS and then stained with solution containing 40 mM citric acid/Na phosphate buffer, 5 mM Potassium hexacyano-ferrate (II) tri-hydrate solution, 5 mM Potassium hexacyano-ferrate (III) solution, 150 mM NaCl, 2 mM Magnesium chloride hexahydrate solution and 1 mg/mL X-gal solution for 16 h at 37°C washed with PBS and viewed under microscope.
Cell cycle analysis by flow cytometry
Cells were fixed with ice-cold 70% ethanol (overnight at −20°C), permeabilised with 0.1% Triton X-100 (in PBS), treated with RNase A and stained with PI. The DNA content was analysed with a flow-cytometer using BD FACS Verse equipped with BD FACS Suite software for data analysis.
Results
Low-dose rotenone exposure influences HeLa cell survival depending upon glucose availability
Several cancerous cell lines are known to mainly utilise glycolysis instead of mitochondrial respiration to generate ATP for their survival and proliferation. Thus, to evaluate ability of HeLa cells to proliferate under different medium conditions, cell viability assay was performed for HeLa cells cultured in HG medium, PG and PGT medium for three consecutive days. Decrease in cell viability was observed for PG medium but to a lesser extent while in PGT medium cell viability decreased by 36% (P < 0.0001) (Figure 1A). Rotenone is a class 5 mitocan that irreversibly binds to complex I allowing the reduction of molecular oxygen to produce oxidant. Evaluation of ROS generation by H2DCFDA oxidation after 72 h revealed more than twofold ROS generation (2.12-fold) by HeLa cells cultured in PGT as compared to 1.6-fold increase in PG (Figure 1B). The outcome of mitochondrial activity determines the intracellular ATP levels. PGT medium showed a significant drop in ATP generation (0.05-fold) as compared to cells grown in PG medium (0.17-fold) measured against HG (Figure 1C). A quantitative analysis of glucose left in the media after 72 h of treatment revealed significant ∼2-fold (0.807 ± 0.12 mg/mL/106 cells) and ∼1.89-fold (0.746 ± 0.09 mg/mL/106 cells) increase in the amount of glucose consumed by PG and PGT-treated HeLa cells, respectively, compared to normal (0.4 ± 0.02 mg/mL/106 cells) HG-treated HeLa cells (Figure 1C). An increase in glucose consumption but corresponding decrease in ATP generation in both PG and PGT indicates lowering in energy production efficiency due to stressed condition of the HeLa cells. Also, it shows that the efficacy of PG condition to induce stress and decrease the viability and metabolic activity in the HeLa cells was enhanced by prolonged low-dose rotenone exposure. Importantly, rotenone treatment in non-limiting glucose condition had no effect on cellular survivability and intracellular ATP level. The actual cause behind this reduction of proliferation by the combined treatment may be differential cell cycle progression and, hence, cell cycle analysis (Figure 1D) was performed, which indicated an increase in percentage of S-phase from 19.18% (±9.63%) in HG medium to 23.78% (±8.32%) in PG and 37.94% (±6.61%) in PGT along with concomitant decrease in percentage of G2/M and G1 population (Figure 1E). A considerable increase in sub-D (sub-diploid) population was observed from 1.62% (±1.65%) in HG to 18.38% (±2.10%) in PG and 38.67% (±2.05%) in PGT (Figure 1E). Thus, major cell population composition in case of PG treatment was that of G1 + S phases while that in case of PGT was S + sub-D phases. This indicated cell growth arrest due to PG and both cytostatic and cytotoxic effect generated due to PGT.
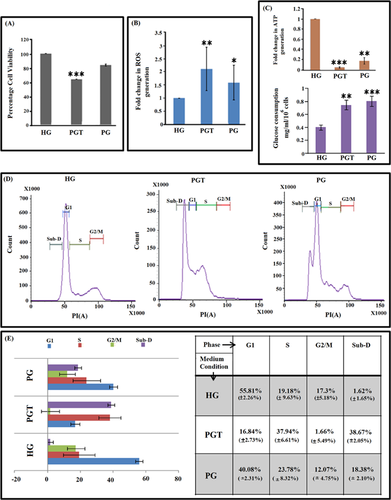
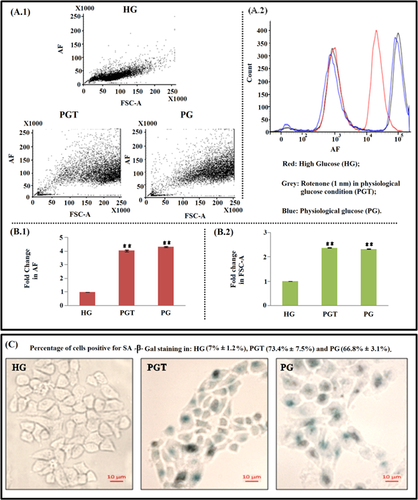
PG condition-induced senescence while PGT-induced co-existing senescence and apoptosis in HeLa cells
In cancerous cells, activated growth-promoting pathways help in cell cycle progression. Inactivation of growth cycle of HeLa cells under physiological glucose condition along with rotenone exposure may force the cells towards senescence and/or apoptosis or both. To assess how HG, PG and PGT medium conditions affect cell cycle progression of HeLa cells, the hallmarks of senescence such as large flat morphology and senescence-associated β-galactosidase activity (Li et al., 2013) were checked. For senescence confirmation, SA-β-gal staining was performed. Senescent cells show increased intrinsic fluorescence, called AF, which is caused by the accumulation of oxidatively damaged proteins and lipids. FACS analysis for AF and cell size (Figure 2A.1) under the three different conditions showed that most of the cells grown in PG and PGT medium shifted to higher level of AF (Figures 2A.2 and 2B.1) and cell size (Figure 2B.2). However, in PGT condition, AF was found to be lesser (insignificant decrease) as compared to that in PG, which indicated towards apoptotic induction (Wolbers et al., 2004). SA–β-Gal staining for ageing pigment lipofuscin showed that cells grown in HG medium had negligible staining (7% ± 1.2%) while those cultured in PG and PGT medium showed intense blue stained cells (66.8% ± 3.1% in PG and 73.4% ± 7.5% in PGT) having flattened and enlarged morphology (Figure 2C). All these features showed that cellular senescence induced in the HeLa cells due to glucose deprivation, a condition present in both PG and PGT media. A minor presence of apoptotic population in the PGT-treated HeLa cells pointed towards a co-existence of senescent and apoptotic populations, a feature that could have arisen due to addition of rotenone in the glucose deprived condition (PGT).
p53 stabilises in HeLa cells due to elevation in ROS and decline in ATP level and accumulates in nuclei
HeLa cells express wt p53, but the protein product is HPV-inactivated due to its degradation by the E6 proteins. The E6 protein encoded by oncogenic HPVs binds p53 and stimulates its degradation (Scheffner et al., 1990). However, increase in ROS is linked with degradation of E6 (Jing et al., 2014) and inhibition of E6 reinstall the function of p53 (Goodwin and DiMaio, 2000). A number of chemotherapeutic compounds that generate oxidative stress are known to reactivate p53 like cisplatin (Wsierska-Gadek and Horky, 2003) which represses E6 oncoprotein. Many compounds like Morinda citrifolia (Gupta et al., 2013) and Taurine (Kim and Kim, 2013) show anti-cancerous property in cervical carcinoma cells, with up-regulation of p53. Besides ROS E6-promoted degradation of p53 is also ATP dependent (Scheffner et al., 1990). In the present study, p53 was found to be expressing in both PG and PGT conditions (Figure 3A), while it was almost undetectable in HG-treated HeLa cells. As both PG and PGT conditions cause considerable drop in ATP generation (discussed earlier), the shutdown of p53 degradation in PG and PGT is fairly possible. p53 has a short life span but gets transiently stabilised and is imported in the nucleus in response to genotoxic stress, hypoxia, nucleotide depletion where it induce or represses downstream target gene that culminates in cell cycle arrest, mitotic catastrophe or apoptotic induction (Gonçalves et al., 2011). The immuno-cytochemical analysis showed most of it localising in the nuclei in the PG and PGT conditions (Figure 3C). Thus, oxidative stress in PG and PGT resulted into ATP depletion responsible for increased stability, nuclear accumulation and detectable expression of p53 in the two stress conditions.
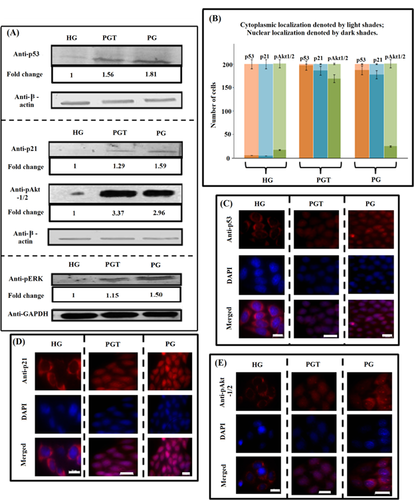
Under PG condition, p53 and p21 up-regulation confirmed premature senescence in HeLa cells
PG and PGT conditions showed increase in oxidative stress and decline in ATP level which led to stabilisation of p53 against degradation by E6 protein. Stabilised p53 plays an important role in triggering downstream target protein p21 (DeFilippis et al., 2003). It is also known that premature senescence induced by oxidative stress is regulated by p53 (Borodkina et al., 2014). It initiates senescence by activating the expression of the cyclin-dependent kinase inhibitor, p21 (Romanov et al., 2010). Therefore, we checked the expression of p53 and p21 and observed maximum increase of p53 (1.81-fold, P < 0.001) and p21 (1.59-fold, P < 0.001) in HeLa cells grown in PG medium (Figure 3A). Activated ERK is known to stabilise p53 (Persons et al., 2000). The level of pERK was found to be maximum (1.51-fold, P < 0.001) in PG (Figure 3A). Localisation pattern of p53 also changed, it was dispersed in the cytoplasm of cells grown in HG medium but was almost exclusively within the nuclei of cells grown in PG and PGT (Figures 3B and 3C), which is in concurrence with many studies (Mesaeli and Phillipson, 2004; Gonçalves et al., 2011). As the function of p21 depends on its subcellular localisation (Piccolo and Crispi, 2012), glucose deficiency leads to nuclear localisation of p21 indicating its role in cell cycle arrest (Figures 3B and 3D). Nuclear localisation of p53 and p21 along with their elevated levels forced HeLa cells towards senescence in glucose deficient condition. These features clearly indicated towards senescence induction in the HeLa cells under PG condition.
In case of PGT-treated HeLa cells, the expression of p53 increased to 1.56-fold (P < 0.001) and it localised in the nuclei (Figures 3A–3C). Expression of p21 increased to 1.29-fold (P < 0.001) and it localised in the nuclei (Figure 3D), indicating growth arrest. However, expression of p53, p21 and pERK did not reach as high as that in PG condition (Figure 3A). This indicated that rotenone present in the PGT medium changed the signalling which could otherwise be directed towards senescence only.
Akt is known to show antagonistic pleiotropic functions, promoting both cell survival (Kandel et al., 2002) and cell death (Nogueira et al., 2008). Its activation is reported to induce ROS-mediated apoptosis in cancer cells (Nogueira et al., 2008) and leads to nuclear accumulation of pAkt1/2 (Nguyen Xuan et al., 2006). Significant increase of pAkt-1/2 in HeLa cells both in PG (2.96-fold, P < 0.001) and PGT (3.37-fold, P < 0.0001) (Figure 3A) was observed. Its localisation was predominantly nuclear in PGT, whereas cytoplasmic localisation prevailed for cells in PG and HG medium (Figure 3E). Maximum increase in p-Akt1/2 expression and its nuclear localisation indicated towards ROS-mediated apoptosis induction in PGT.
PGT medium condition induced apoptosis and senescence in HeLa cells mediated by up-regulation of NFκB
Oxidative stress has been reported to both activate (Schreck et al., 1991) as well as repress NFκB (Zmijewski et al., 2007). Here, we observed that ROS generated due to glucose depletion (PG) down-regulated NFκB expression (0.48-fold, P < 0.001) but in PGT medium condition its up-regulation took place (1.23-fold, P < 0.001) (Figure 4A). NFκB, at a low level of expression, is located predominantly in the cytoplasm but at higher level nuclear localisation prevails (Zabel et al., 1993). Cytoplasmic localisation of NFκB was observed in PG and HG but nuclear localisation predominated in PGT (Figure 4B). As ROS often function in multiple places within a given pathway, and sometimes in opposing ways such seems to be the case with regard to ROS functions in the NFκB pathway in this scenario. Increased expression of NFkB and a simultaneous decrease in p53 expression shows the two transcription factors working in opposition of each other which is in concurrence with many studies (Webster and Perkins, 1999). Nuclear localisation of NFκB activated apoptotic proteins Bax (1.23-fold, P < 0.001) and caspase-3 (1.17-fold, P < 0.001) on rotenone exposure confirming apoptotic induction due to this combination strategy. The immuno-cytochemical analysis also revealed caspase-3 localising in the cytosol under PGT (Figure 4C), a feature of apoptotic signalling (Zhivotovsky et al., 1999).
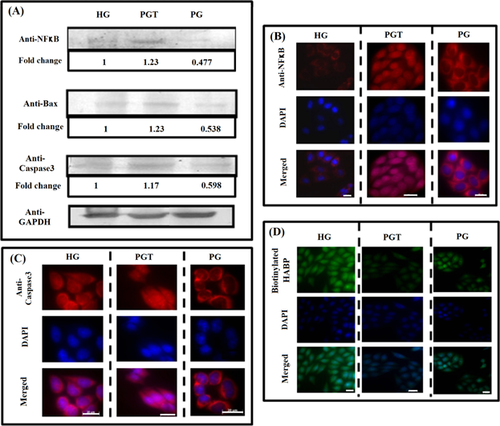
Lowering in p53 expression brought about due to increased expression of NFκB in PGT did not lead to an expected decrease in growth arrested population due to p21 down-regulation. Instead, there was an increase in the S-phase population in PGT as compared to PG. This could be attributed to the multifunctional nature of NFκB, which has been found to enhance senescence also in many cases (Chien et al., 2011; Rovillain et al., 2011). Thus, the primary stress response towards PG which was p53 and p21-mediated growth arrest switched to NFκB-mediated apoptosis and senescence in PGT condition.
Tumorigenicity loss in HeLa cells under PG and PGT treatment
Tumour growth and development is influenced by its microenvironment and extra cellular matrix (ECM). Hyaluronan (HA), an important component of ECM, is known to increase during cancer progression and malignant transformation (Itano et al., 2008). Owing to its unique hydrodynamic properties and by interaction with hyaladherins, it influences cancer cell signalling (Stern, 2008). Interestingly, generation of excess ROS is known to degrade HA leading to apoptosis (Uchiyama et al., 1990). The immuno-cytochemical analysis showed decline in level of HA both in PG and PGT, confirming the loss of tumorigenicity in HeLa cells. However, the decrease was maximal in case of PGT-treated cells (Figure 4D).
Discussion
Several studies have shown that aerobic glycolysis and oxidative metabolism interchange during the proliferation and transformation of cancer cells (Moreno-Sanchez et al., 2007) in which mitochondria might represent the centre of cellular ATP production as well as regulator of apoptosis. As cancer cells mitochondria hold a strong interconnection between metabolism and apoptosis, they may be a good therapeutic hit.
In this report, we utilised the metabolic alteration, namely hyperglycolytic phenomenon (Warburg effect) of HeLa cell line and mitochondrial dysfunction, as target for synergistic effect on control of cancer progression. To target combination effects, aimed to specifically kill the cancer cells, herein, HeLa cells were grown in limited glucose availability with very low dose of complex I blocker, rotenone. It showed growth retardation; fall in ATP generation with induction of ROS and cell cycle arrest. Notably, glucose depletion induced senescence in HeLa cells, which was identified with auto-florescence, an increase in cellular size and SA-ß-gal staining. Combination treatment with physiologically low-dose rotenone triggered apoptotic signalling cascade confirming that in glucose shortage condition these cells are forced to depend on mitochondria which makes them more sensitive to inhibitors of mitochondrial functions.
A 50 μМ rotenone treatment in medium containing 11 mM glucose is effective in induction of growth retardation as compared to 55 mM glucose medium in Jurkat T Leukemia cells as shown by Mendivil-Perez et al. (2014). In this study, we used medium containing 5.5 mM glucose (PG) in which non-glycolytic normal cells proliferate in vivo against 25 mM HG medium in which cancer cells are generally grown (in vitro), due to their high-glucose requirement. Along with PG condition prolonged (72 h) low-dose exposure to rotenone (1 nM) was maintained, which alone does not induce growth retardation in normal cells (data not shown). Exposure of rotenone with PG decreased intracellular ATP generation level even when glucose consumption increased indicating a decreased efficiency in energy production in PGT. Since activation of Akt is known to be involved in energy metabolism and also regulates intracellular ROS level, substantial activation of pAkt1/2 in PG along with its nuclear localisation confirmed the cellular starvation stage. In this scenario, PG condition sensitised cells to oxidative stress-induced premature senescence as evidenced by increase in FSC-A, AF and intense positive SA-ß-gal staining as compared to cells in HG medium. p53, which is normally degraded in the HeLa cells, was also found to get stabilised due to its nuclear localisation and depleted ATP pool in both PG and PGT and hence reached detectable levels of expression. The level of pERK was also maximal in cells in PG condition which is again known to stabilise p53. p53 is known to regulate premature senescence by activating cyclin-dependent kinase inhibitor p21. Expression of p53 and p21 was found to be maximal in PG medium in absence of rotenone. Moreover, both got localised in the nucleus in PG medium. Cell cycle analysis showed arrest of cells in G1 and S phases in PG condition and decrease in percentage of cells in G2 phase. The percentage of sub-diploid population indicative of apoptosis was maximal in PGT medium. A marked increase in S-phase population was also observed in PGT. The induction of p21 by p53-dependent mechanism arrested cell cycle and blocked HeLa cells in G1 and S phases of cell cycle in PG. p21 also acts as an inhibitor of apoptosis and arrests cell cycle so is the case in PG medium in absence of rotenone.
Expression of NFκB is regulated by oxidative stress. Its expression decreased in PG condition but increased in PGT condition due to increased oxidative stress. Elevated NFκB increased expression of pro-apoptotic protein Bax. NFκB has been reported to negatively regulate p53 also and hence, upon increase in NFκB expression, p53 was down-regulated. Activation of NFκB, along with decrease in level of p53, p21 and ultimately increased expression of caspase-3, induced programmed cell death. The level of Bax and Caspase-3, key markers of apoptosis reduced in PG condition in absence of rotenone but increased on exposure of cells to low dose of rotenone, clearly indicating apoptotic induction due to additional effect of rotenone. Senescence induction in case of PGT-treated HeLa cells is also mainly attributed to increased expression and nuclear localisation of NFκB.
A decline in level of HA in both PG and PGT was found. HA expression reached minima in the PGT condition and this also supported apoptotic induction. Remarkably, all our findings strongly pointed towards growth arrest of HeLa cells in PG medium due to senescence, which with rotenone (PGT) further enhanced the cyto-static effect and also triggered cell death by apoptosis.
Thus, present study suggested combined therapy using rotenone with regulated glucose availability may be an important therapeutic approach, as surrounding normal cells might have little response compared to cancer cells. Therefore, strategies involving the manipulation of both glycolytic and mitochondrial pathways might efficiently eradicate cancer cells.
Acknowledgements and funding
We sincerely thank Professor Kasturi Datta for her scientific inputs. The author(s) received financial support from ICMR (Indian Council of Medical Research), CSIR (Council of Scientific and Industrial Research), UGC (University Grant commission) and DST (Department of Science and Technology) funding from Government of India for the research, but no financial support for publication of this article.
Authors’ contributions
NRA participated in the design of the study, carried out all experiments related to cellular treatment and growth analysis, acquisition of data, and analysis and drafted the manuscript. NM carried out the FACS and related statistical analysis with respect to growth and helped to draft the manuscript. JSP participated in conducting glucose estimation of media and also in Western blot analysis. IG conceived the study, participated in its design and coordination and revised the manuscript critically for important intellectual content before giving the final approval of the version to be published. All authors have read and approved the final manuscript.
Conflicts of interest
The authors declare no conflicts of interest.




