Oleanolic acid induces autophagic death in human gastric cancer cells in vitro and in vivo
Abstract
Oleanolic acid (OA), a plant-derived pentacyclic terpenoid, is known to have hepatoprotective effects. In this study, we found that OA induced autophagic cell death in multiple human gastric cancer cell lines. Moreover, OA-induced autophagy was shown for the first time in human gastric cancer cells, evidenced by the formation of GFP-RFP-LC3 puncta and autophagosomes. OA suppressed phospho-mTOR through inhibition of the PI3 K/AKT and ERK/p38 MAPK signalling pathways and through activation of the AMPK signalling pathway. Furthermore, we found that OA-induced cytotoxicity and autophagy could be blocked by the autophagy inhibitor 3-methyladenine or via siRNA targeting Beclin-1. Our in vivo research showed that OA delayed the formation of MGC-803 tumours in an autophagy-dependent manner. These results reveal a novel mechanism for OA in gastric cancer cells and suggest that OA could be a novel agent in the treatment of gastric cancer.
Abbreviations
-
- 3-MA
-
- 3-methyladenine
-
- GFP
-
- green fluorescent protein
-
- LC3
-
- microtubule-associated protein 1 light chain 3
-
- MTT
-
- 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
-
- OA
-
- oleanolic acid
-
- RFP
-
- red fluorescent protein
-
- siRNA
-
- small interfering RNA
-
- TEM
-
- transmission electron microscopy
Introduction
Oleanolic acid (OA) is a plant-derived pentacyclic terpenoid that is widely distributed in various medicinal plants, fruits and herbs (Park et al., 2014). In China, OA has been used clinically as an anti-hepatitis drug for more than 20 years. In addition to its excellent hepatoprotective effect, OA has attracted considerable attention due to its medicinal value as an anti-inflammatory, antioxidant, immunomodulatory and anti-HIV compound (Takemura et al., 2011; Cheng et al., 2015). Recent studies have indicated that OA and its derivatives possess potent cancer-preventing properties. OA induces apoptosis in various breast cancer cells though either the extrinsic or intrinsic cell death pathway (Akl et al., 2014). In addition to apoptosis, OA blocks the proliferation of human leukaemia K562 cells by controlling the transition from the G1 to S phase (Ng et al., 2013). In human liver cancer Huh7 cells, OA down-regulates NF-κB, which is a protein that is responsible for cytokine production and cell survival (Shyu et al., 2010). In a rodent model, OA was shown to prevent 1, 2-dimethylhydrazine-induced colon carcinoma (Furtado et al., 2008). One phase I dose-escalation study enrolled patients with advanced solid cancers to determine the toxicity of OA derivatives (Speranza et al., 2012). However, the mechanism by which OA prevents human gastric cancer remains largely unknown.
Eukaryotic cells employ autophagy as a means to survive nutrient deprivation and the absence of growth factors. Autophagy is characterized by lysosomes contributing to the degradation of intracellular components, including proteins, organelles and even pathogens that reach the cytosol after cell invasion (Cuervo and Macian, 2012). Autophagy can also play an active role in both survival and stress-induced instances of cell death (Galluzzi et al., 2014). Recently, several studies reported that some natural products isolated from herbs could stimulate excess autophagy in cancer cells, leading to cell death (Meschini et al., 2008; Aryal et al., 2014). Therefore, natural products from medicinal herbs that trigger autophagic cell death may represent potent anticancer agents for the treatment of gastric cancer.
In this study, we sought to identify the effects of OA on human gastric cancer cells and further examine the cell signalling pathway. We observed that the OA-induced cytotoxicity in human gastric cancer cells was attributed to autophagy. In addition, our data showed that OA inhibited MGC-803 xenograft growth in BALB/c nude mice through an autophagy pathway. These preclinical studies suggest that OA could be a potential medicine for the treatment of gastric cancer.
Materials and methods
Chemicals and reagents
OA, 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), 3-methyladenine (3-MA) were purchased from Sigma. Antibodies specific for LC3B, Beclin-1, mTOR, phospho-mTOR, AKT, phospho-AKT, AMPK (CST, 5831), phospho-AMPK, ERK1/2, phospho-ERK1/2, PTEN, phospho-PTEN, phospho-ULK1, phospho-PI3 K, phospho-p38, and β-actin were purchased from Cell Signalling Technology. A secondary antibody conjugated with Alexa Fluor680 was purchased from Jackson ImmunoResearch Laboratories. All other chemicals were of the highest purity available.
Cell lines and cell culture
The human normal gastricepithelial cell line GES-1 and RGM-1, human gastric cancer cell lines SGC-7901, MGC-803 and BGC-823 obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). All cell lines were routinely cultured in RPMI 1640 medium (Gibco), which contained 10% fetal calf serum (Gibco), 100 U/mL penicillin, and 100 U/mL streptomycin, in a humidified cell incubator at 37°C with an atmosphere of 5% CO2.
Cell viability assay
Cell viability was measured by the MTT method. Briefly, exponentially growing cells were seeded in 96-well plates, then incubated with at various concentrations of OA for 24, 48, or 72 h. MTT was then added to each well, and the cells were incubated for a further 4 h at 37°C. The formazan precipitate was dissolved in 150 µL DMSO, and the absorbance at 490 nm was measured using a microplate reader (Thermo). Each test was repeated at least three times.
Western blot analysis
Approximately 30 µg of lysed protein was separated by 10–15% sodium dodecyl sulphate PAGE, transferred to a nitrocellulose blot membrane (Bio-Rad), and then blocked for 1 h in blocking buffer (5% bovine serum albumin solution and 0.1% Tween 20 in Tris-buffered saline (TBST)). The membrane was incubated with the primary antibody overnight at 4°C. After three washes, the blots were subjected to a secondary antibody for 1 h in blocking buffer. Proteins were visualised using an Odyssey Imager (LI-COR).
GFP-RFP-LC3transfection and fluorescence
The cells were transfected with the plasmid GFP-RFP-LC3 using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. At 48 h after transfection, cells were treated with OA for 24 h. The fluorescence of GFP-RFP-LC3 was observed; autophagosomes (yellow dots) and autolysosomes (free red dots) in each cell were counted under an Olympus FV1000 confocal microscope. The GFP-RFP-LC3 plasmid was kindly provided by Prof. Mao Xiang (Sun et al., 2012).
Transmission electron microscopy (TEM)
The collected cells were fixed overnight in 2.5% glutaraldehyde in phosphate buffer (0.1 M, pH7.0). After three washes, the specimens were fixed with 1% OsO4 for 1 h. The specimen was dehydrated using a graded series of ethanol (30, 50, 70, 80, 90 and 100%) for 15 min at each step and then transferred to pure acetone for 20 min. The specimen was placed in a 1:1 mixture of pure acetone and final resin mixture for 1 h, transferred to a 1:3 mixture of pure acetone and final resin mixture for 3 h, and then to the final spur resin mixture overnight. Specimens were heated at 37°C for more than 9 h. Finally, the specimens were sectioned with a LEICA EM UC7 ultramicrotome. Sections were stained with uranyl acetate and alkaline lead citrate for 5 min and observed with a Hitachi Model H-7650 TEM.
Transient transfection with small interfering siRNA
siRNA targeting Beclin-1 and CypD were purchased from Santa Cruz. Cells were transfected with the corresponding siRNA of the target gene or scrambled control siRNA by Lipofectamine RNAiMAX (Invitrogen), according to the manufacturer's instructions. Knockdown efficiency was assessed by Western blot after 48 h of transfection.
Xenograft model in nude mice
The procedure of establishing MGC-803 xenografts in nude mice was approved by the Zhejiang University Laboratory Animal Centre (Hangzhou, China). Briefly, 4 × 106 MGC-803 cells were subcutaneously injected into the right flanks of 6-week-old female nude BALB/c mice, which were then randomly divided into three groups (n = 8). Beginning at day 7, the MGC-803 + OA group was orally treated with OA at 100 mg/kg body weight every day. The MGC-803 + OA + 3-MA group was intraperitoneally injected with 10 mM 3-MA in 50 µL of DMSO at 3-day intervals and received oral OA treatment as well. The MGC-803 group was injected with 50 µL of DMSO as acontrol. Tumour diameters were measured with a calliper at 3-day intervals beginning at day 7. Tumour volume = (shortest diameter2 × longest diameter)/2. All of the mice were sacrificed on day 37, and tumours were harvested.
Statistical analysis
Data are presented as the means ± standard deviations (SD). Statistical analyses were performed by one-way analysis of variance (ANOVA) followed by Tukey's post hoc test. A value of P < 0.05 was considered statistically significant.
Result
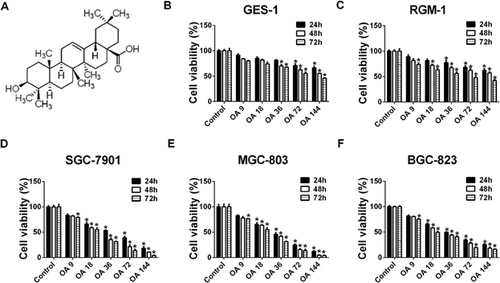
OA inhibits the growth of human gastric cancer SGC-7901, MGC-803 and BGC-823 cells
To identify the potential of OA for treating human gastric cancer, GES-1, RGM-1, SGC-7901, MGC-803 and BGC-823 cells were treated with OA, and cell viability was determined by the MTT assay. We found that OA inhibited the growth of SGC-7901, MGC-803 and BGC-823 cells in a dose- and time-dependent manner (Figures 1D and 1F). The 50% inhibitory concentrations (IC50) for SGC-7901 cells were 39.5, 25.9 and 21.2 µM after treatment with OA for 24, 48 and 72 h, respectively. The IC50 values in MGC-80-3 cells and BGC-823 cells after treatment with OA were 30.6 and 39.4 µM (for 24 h), 24.0 and 30.1 µM (for 48 h), 20.4 and 22.4 µM (for 72 h), respectively. In contrast, only a small percentage of inhibition was detected in OA-treated GES-1 and RGM-1 cells (Figures 1B and 1C).
OA induces autophagy in human gastric cancer SGC-7901, MGC-803 and BGC-823 cells
Beclin-1 controls the initiation of autophagocytosis and activates microtubule-associated Protein 1; LC3, whose cytosolic form (LC3-I) is lipidated to the LC3-II form during autophagy, is a specific marker for autophagosome formation. As shown in Figure 2A, compared with the untreated group, OA induced a noticeably greater amount of Beclin-1 and LC3-II in SGC-7901, MGC-803 and BGC-823 cells after OA treatment for 24 h.
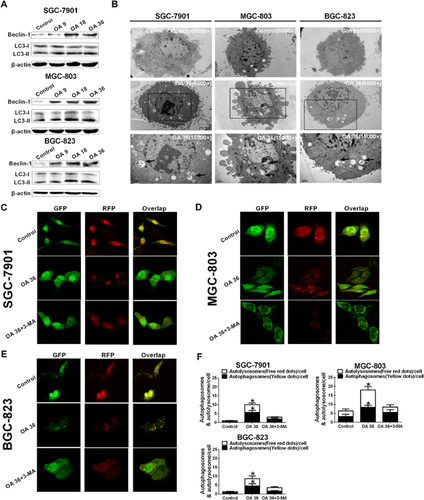
To confirm the characteristics of autophagy induced by OA, the accumulation of GFP-RFP-LC3 puncta, autophagosomes and autolysosomes in SGC-7901, MGC-803 and BGC-823 cells was investigated by observation with transmission electron microscopy (TEM) and confocal microscopy. OA-treated SGC-7901, MGC-803 and BGC-823 cells showed cytoplasmic accumulation of autophagosomes, a morphological marker of autophagy as determined by TEM, after OA treatment for 24 h (Figure 2B). In addition, confocal microscopy analysis revealed that, in contrast to the relatively homogeneous distribution in control cells, GFP-RFP-LC3 puncta accumulated in the cytoplasm of OA-treated cells (Figures 2C–2E). Autophagosomes (yellow dots) and autolysosomes (free red dots) in each cell were counted as shown in Figure 2F. Pretreatment with 2 mM 3-methyladenine (3-MA, an autophagy inhibitor) for 2 h obviously suppressed the accumulation of autophagosomes and autolysosomes (Figures 2C–2F). These data indicate that autophagy is induced by OA in SGC-7901, MGC-803 and BGC-823 cells.
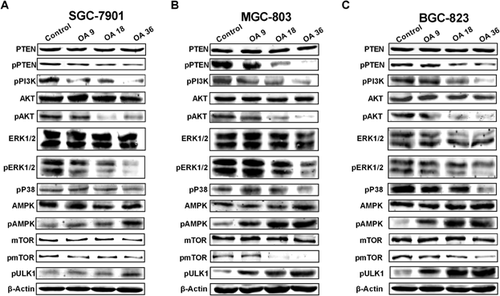
OA induces the inhibition of mTOR
mTOR has been reported to be involved in the cell autophagy process, and the suppression of mTOR phosphorylation is considered to be responsible for the early triggering of autophagy, which is regulated by the PI3 K/AKT, ERK/MAPK and AMPK signalling pathways (Dunlop and Tee, 2014; Shimobayashi and Hall, 2014). We investigated the role of mTOR in OA-induced autophagy. As shown in Figure 3, OA treatment for 24 h decreased the phosphorylation of PI3 K, AKT, ERK1/2, p38 and mTOR, but increased the phosphorylation of AMPK. ULK1, a kinase essential for autophagy initiation, was controlled by phospho-mTOR, OA treatment increased phosphorylation of ULK1 indicated an increasing level of autophagic initiation. These data suggest that OA induces autophagy by suppressing phospho-mTOR through inhibition of the PI3 K/AKT and ERK/p38 MAPK signalling pathways and activation of the AMPK signalling pathway.
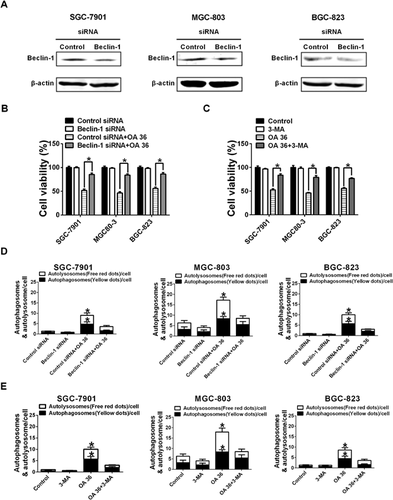
Inhibition of autophagy attenuates OA-induced cytotoxicity
Because Beclin-1 controls the initiation of autophagocytosis, suppressing Beclin-1 expression may attenuate OA-induced cytotoxicity and autophagy. We found that knocking down the expression of Beclin-1 in SGC-7901, MGC-803 or BGC-823 cells by siRNA led to a reduction in OA-induced cell death, autophagosomes and autolysosomes (Figures 4A, 4B and 4D). To further examine whether the OA-induced cytotoxicity of SGC-7901, MGC-803 and BGC-823 cells was caused by autophagy, we examine the suppression of OA-induced cytotoxicity and autophagy via the autophagy inhibitor 3-MA. As illustrated in Figures 4C and 4E, a 2 h pretreatment with 2 mM 3-MA significantly reduced the OA-induced cytotoxicity and accumulation of GFP-RFP-LC3 vacuoles in SGC-7901, MGC-803 and BGC-823 cells. Taken together, our data indicate that OA-induced cytotoxicity in SGC-7901, MGC-803 and BGC-823 cells is caused by autophagy.
Growth of human gastric cancer MGC-803 cells in nude mice is inhibited by OA treatment
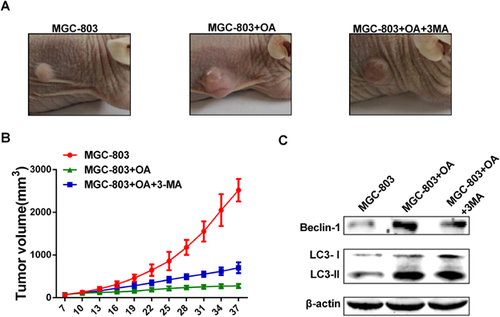
We applied the tumour xenograt mouse model of MGC-803 cells to investigate the therapeutic potential of OA in gastric cancer in vivo. As shown in Figures 5A and 5B, in the drug-treated mouse group, OA decreased the growth of MGC-803 tumours and delayed the occurrence of tumour formation compared with the control. 3-MA treatment abolished the inhibitory effect of OA on tumour growth. After sacrificing the mice and observing tumour formation, we confirmed that OA-induced autophagy was decreased in 3-MA-treated MGC-803-originating tumours by examining the expression of LC3-II and Beclin-1 (Figure 5C).
Discussion
Oleanolic acid is a ubiquitous pentacyclic multifunctional triterpenoid that is widely found in several dietary and medicinal plants. Natural and synthetic OA derivatives can modulate multiple signalling pathways including NF-κB, AKT, signal transducer and activator of transcription 3, mammalian target of rapamycin, caspases, intercellular adhesion molecule 1, vascular endothelial growth factor, and poly(ADP-ribose) polymerase, in a variety of tumour cells (Shanmugam et al., 2014). However, the mechanism by which OA inhibits human gastric cancer cells remains largely unknown. In this study, we found that OA effectively inhibited cell proliferation in a dose- and time-dependent manner in human gastric cancer SGC-7901, MGC-803 and BGC-823 cells. Investigating the mechanism by which human gastric cancer cells undergo suppression of cellular proliferation in response to OA treatment, we found that OA induced autophagy in human gastric cancer cells. Moreover, OA-induced autophagy and cytotoxicity was largely attenuated by the autophagy inhibitor 3-MA or Beclin-1 siRNA.
Autophagy has a vital role in cellular development, differentiation, survival and homeostasis and is the most important mechanism for the degradation and recycling of long-lived proteins, protein aggregates, organelles and intracellular pathogens (Orvedahl and Levine, 2009). During normal physiologic conditions, autophagy helps to maintain cellular homeostasis by “clearing” dispensable/damaged intracellular structures, thus providing a crucial tool for protection against the accumulation of toxic cellular components (Marino et al., 2012). Thus, autophagy serves as a protective response to various stress stimuli as suggested by the association between defective autophagy and several human pathologies, including cancer (Meijer and Codogno, 2009). Because the identification of metabolic transformation is an important determinant of cancer progression, the role of autophagy in cancer development and the significance of autophagy in anticancer therapeutics have not been absolutely demonstrated (Mizushima and Komatsu, 2011). Autophagy is usually regarded as having a positive effect on cell health through the removal of potentially harmful protein aggregates and damaged organelles. To adapt to the adverse conditions induced by stress from anticancer therapies, cancer cells may trigger an autophagic response to engulf a portion of the cytoplasm and organelles into autophagic vesicles as part of the survival response to stress (Degenhardt et al., 2006; Macintosh and Ryan, 2013). In this case, autophagy may be a survival mechanism in cancer cells. On the other hand, autophagy can also be considered a form of programmed cell death, termed “autophagic cell death”. In this regard, autophagy might be a vital mechanism for cancer cell death by these agents. Determining whether OA-induced autophagy in SGC-7901, MGC-803 and BGC-823 cells is a survival mechanism or a cell death mechanism was a key aim of this study. Our results indicated that 3-MA (an autophagy inhibitor) could attenuate the cytotoxicity of OA, suggesting that OA causes autophagic cell death. These findings were further validated by the knockdown of Beclin-1, an adaptor protein that is critical for autophagosome formation (Salminen et al., 2013). The ability of OA to trigger multiple death pathways suggests its effectiveness and versatility in killing cancer cells.
In this study, we have shown that OA induces autophagy via effects on mTOR. As a central signal integrator, mTOR has a crucial role in the regulation of metabolism and cell growth via receiving signals from nutrients, growth factors and many cellular kinases, including AMPK.44 AMPK phosphorylation activates downstream signalling that leads to mTOR inhibition and triggers autophagy (Shaw, 2006), which is consistent with the function of AMPK in initiating catabolic processes.46 Additionally, we showed that OA treatment has a stronger effect on the inhibition of the PI3 K/AKT and ERK/p38 MAPK signalling pathways. Constitutive activation of mTOR by oncogenic signalling via PI3 K/AKT or ERK/p38 MAPK in cancer cells supports the tumour suppressive function of autophagy (Shaw and Cantley, 2006; Cairns et al., 2011). Taken together, these data indicate that OA suppresses phospho-mTOR via inhibiting the PI3 K/AKT and ERK/p38 MAPK signalling pathways and activating the AMPK signalling pathway.
We applied a tumour xenograft mouse model of MGC-803 cells to investigate the therapeutic potential of OA in gastric cancer in vivo. These data indicated that OA decreased the growth of MGC-803 tumours and delayed the occurrence of tumour formation. 3-MA treatment abolished the inhibitory effect of OA on tumour growth. Moreover, the expression of LC3-II and Beclin-1 in OA-treated MGC-803-derived tumours was enhanced, but this expression was attenuated by 3-MA treatment. Taken together, these observations indicate that OA can suppress the growth of MGC-803 cells in vivo by inducing autophagy.
In conclusion, OA induced apoptosis-independent autophagic cell death in multiple human gastric cancer cells via modulation of the expression of phospho-mTOR, which is associated with the inhibition of the PI3 K/AKT and ERK/p38 MAPK signalling pathways and the activation of the AMPK signalling pathway. OA effectively decreased the growth of MGC-803 tumours in a nude mouse model. The results contribute to our understanding of OA and provide clear evidence for its promising potential in preclinical and clinical situations.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgements and funding
We thank the highly qualified native English-speaking editors (Taylor & Francis Editing Services) for editing work. This work was supported by grant 81302680 from the National Natural Science Foundation of China, 2015M580518 from the Postdoctoral Science Foundation Project of China, BSH1502123 from the Postdoctoral Science Foundation Project of Zhejiang Province.




