TGF-β1 promotes bovine mammary fibroblast proliferation through the ERK 1/2 signalling pathway
Abstract
The abnormal proliferation of bovine mammary fibroblasts (BMFBs) impairs mammary gland development and lactation. Severe manifestations develop into breast fibrosis, leading to the culling of cows and causing serious losses to the dairy industry. Transforming growth factor β1 (TGF-β1) is an important modulator of cell proliferation and extracellular matrix formation; however, limited information is available on BMFBs. In this study, a convenient and stable culture method for BMFBs was established. Treatment with 5 ng/mL of TGF-β1 significantly promoted the proliferation of BMFBs and accelerated the cell cycle. TGF-β1 stimulation for up to 12 h significantly increased the relative ERK1/2 mRNA expression and enhanced the protein expression of p-ERK1/2 and cyclin D1. Conversely, the ERK1/2 inhibitor PD98059 blocked these TGF-β1 effects. Further exploration using a mouse model showed that TGF-β1 significantly increased the proportion of fibroblasts and accelerating the cell transition from the G1 to G2/M phases. In addition, TGF-β1 enhanced the expression of fibrosis markers, α-SMA and I Collagen, which could be blocked efficiently by the PD98059 in mouse mammary gland. Finally, immunofluorescence analysis confirmed that TGF-β1 promoted fibroblast proliferation in healthy dairy cows after normal long-term dietary corn straw roughage supplementation. It is suggested that the diet may promote mammary fibroblast proliferation by raising the level of TGF-β1. Our study provides new insights into how nutrition causes undesirable changes in mammary gland structure.
Abbreviations
-
- BMEC
-
- bovine mammary epithelial cells
-
- BMFB
-
- bovine mammary fibroblasts
-
- CS
-
- roughage corn stover
-
- ECM
-
- extracellular matrix
-
- MF
-
- alfalfa
-
- TGF-β
-
- transforming growth factor β
Introduction
The abnormal proliferation of fibroblasts causes an accumulation of extracellular matrix (ECM) proteins. In severe cases, the excessive accumulation of connective tissue is associated with the formation of fibrosis, which leads to tissue and organ dysfunction (Scotton and Chambers, 2007; Zeisberg et al., 2007). Mammary gland fibrosis is common in cows and causes serious losses to the dairy industry. However, the mechanism underlying mammary fibrosis is unclear. Thus, it is of paramount importance to elucidate the molecular basis of the signalling pathways contributing to the abnormal activation of fibroblasts to effectively prevent bovine mammary gland fibrosis.
Transforming growth factor β (TGF-β) is a multifunctional cytokine that plays multiple regulatory roles in different cell types. TGF-β1 plays an important role in the mammary gland development, differentiation and degeneration processes (Moses and Barcellos-Hoff, 2011). As the structural cells of the mammary gland, fibroblasts support the epithelial cells and influence the growth of mammary epithelial cells; additionally, fibroblasts influence the formation of the gland bubble and its differentiation (Cunha and Hom, 1996; Sasaki and Enami, 1999). Current studies have concentrated on the regulatory role of TGF-β1 on epithelial cells during the process of mammary gland remodelling (during the dry period; Vries et al., 2011). In contrast, limited information is available concerning the role and mechanism underlying the regulation of bovine mammary fibroblasts (BMFBs) by TGF-β1. In addition, TGF-β1 is the most potent mediator of fibrosis, promotes fibroblast differentiation into myofibroblasts, stimulates the synthesis of ECM and inhibits ECM degradation. The fibrosis is commonly identified by expression of α-smooth muscle actin (α-SMA) and collagen I (Lasky and Brody, 2000).
In the present study, we isolated primary BMFBs in vitro. We subsequently tested the role of TGF-β1 on mammary fibroblasts and explored the signal transduction mechanisms underlying the promotion of fibroblast proliferation by TGF-β1. We also investigated the effect of TGF-β1 on mammary fibroblast in mice and cows in vivo. Our study not only provides a theoretical basis for the artificial control of mammary gland development and lactation but also provides a foundation for the prevention of mastitis and other breast diseases.
Materials and methods
Reagents and antibodies
The main primary antibodies used in this study were specific for vimentin (BIOSS, bs-8533R), beta-actin (affinity, AF7018), pERK1/2 (Cell Signaling Technology, 9012), cyclin D1 (Abcam, ab95281) α-SMA and I Collagen (BIOSS, bs-0189R and bs-0578R). The HRP- and FITC-labelled goat anti-rabbit secondary antibodies were purchased from the Proteintech group. PD98059 was purchased from Abcam, and TGF-β1 was purchased from PeproTech. Bovine TGF-β1 ELISA Assay Kit was purchased from BD.
Bovine mammary cell culture and treatment
Bovine mammary tissues were collected from healthy lactating Chinese Holsteins. Sterile operation methods were used to collect mammary tissue samples. Bovine mammary epithelial cells (BMECs) and BMFBs were isolated and purified based on differences in their endurance times following trypsin exposure and the durations required for adherence (Farr et al., 1996). Keratin-18 and vimentin antibodies were used to determine cell purity. Isotype control is used to confirm the specific binding of the primary antibody. Fibroblasts were grown in DMEM/F12 with 10% FBS until they were ∼80% confluent. The cells were synchronized by incubation in DMEM/F12 containing 1% FBS for 24 h, followed by treatment with TGF-β1 (5 ng/mL) in the absence or presence of PD98059 (12.5 μM).
MTT to detect cell proliferation
Synchronized, subconfluent fibroblasts were treated with 0, 0.1, 1, or 5 ng/mL TGF-β1 in FBS (−) and 1% FBS or cell-free media as controls. Then, 20 μL of MTT (5 mg/mL) was added to 24-well plates after culturing for 24, 48, or 72 h. After an additional 4 h of incubation, 150 μL of DMSO was added to the wells. The cultures were shocked at 37°C for 10 min prior to measuring the OD490. Each experiment was conducted in triplicate independent experiments.
Flow cytometry
Approximately 2 × 105 cells were collected in 1.5 mL centrifuge tubes after digestion by pancreatic enzymes and then centrifuged at 1,000g for 5 min. The samples were fixed with 70% alcohol for 40 min. PBS buffer was used to wash the cells three times, and then a 500 µL mixture containing 50 µg/mL PI (propidium iodide), 100 µg/mL RNase A, and 0.2% Triton X-100 was added. The cells were resuspended and incubated at 4°C for 30 min in the dark. A flow cytometer (Becton Dickinson, San Jose, CA) was used to detect cell cycle changes. Breast tissues were cut up, digested with collagenase at 37°C and filtered into a single cell suspension. Then, the sample was centrifuged. All other steps were the same as those described for the cultured cells. All experiments were performed in triplicate.
Real-time PCR analysis for gene expression
Total RNA was isolated from BMFBs (bovine mammary fibroblast cells) using TRIzol reagent according to the manufacturer's instructions. Total RNA was treated with DNase I (Sigma) to remove genomic DNA and then quantified at 260/280 nm by spectrophotometry. First-strand cDNA was synthesized with a reverse transcription kit (Takara) using the conditions recommended by the manufacturer. For quantitative analysis of mRNA expression, the FastStart Universal SYBR Green Master kit (Roche) was used to amplify the target genes, and the reactions were performed with the Applied Biosystems Prism 7500 sequence detection system. β-Actin was used as an internal control. The reaction conditions were as follows: 95°C for 10 min, 40 cycles of 95°C for 15 s and 60°C for 1 min, followed by a melting curve. ERK mRNA levels were normalized to β-actin mRNA levels, and the data were analysed using comparative CT. The primers used were as follows: ERK sense 5′-GACGCAACACCTCAGCA-3′ and antisense: 5′-GCCAAGCCAAAGTCACAG-3′, and β-actin sense: 5′-GCCCTGAGGCTCTCTTCCA-3′ and antisense: 5′-GCGGATGTCGACGTCACA-3′.
Western blot analysis for protein expression
For total protein extraction, the cells were washed with cold PBS and then lysed in 100 μL of RIPA buffer with protease inhibitor cocktail. The protein samples were mixed with 5× SDS sample buffer and then subjected to 12% SDS–PAGE. After electroblotting onto PVDF membranes, the membranes were blocked in 5% non-fat milk and incubated with different primary antibodies at 4°C overnight. The following day, the membranes were washed three times with 1× PBS containing 0.1% Tween-20 (PBST) and then incubated with HRP-conjugated secondary antibodies (diluted 1:1,000) for 1 h at room temperature. The immunoreactive proteins on the membranes were incubated with ECL reagent. Protein bands were detected by chemoluminescent imaging system (Thermo Scientific, Pittsburgh, PA). Densitometric analysis was conducted with Image J software. β-actin was used as the loading control.
Immunohistochemistry analysis
The mammary tissues were fixed in 4% PFA and embedded in paraffin. Then, 5 mm sections were cut for histological and immunostaining analysis. The tissues were stained by standard immunostained to study specific proteins. For immunostaining, the tissues were deparaffinized in xylene and rehydrated using decreasing concentrations of ethanol. Antigen retrieval involved microwaving the slides in 10 mM sodium citrate (pH 6) for 20 min. Five percent BSA plus 0.5% Tween-20 in PBS was used as a block and diluent for the antibodies. The sections were incubated overnight with primary antibodies and for 1 h with secondary antibodies. For immunofluorescence, the nuclei were stained with DAPI. The primary antibodies included vimentin (1:100, BIOSS), TGF-β1 (1:200), I Collagen and α-SMA (1:70, BIOSS). The secondary antibody was FITC-labelled goat anti-rabbit. Negative controls were run in parallel using isotype-specific antibodies. The Image pro plus software was used to semi-quantitative the average optical intensity of vimentin positive cells. The average optical density = IOD/Area. The percentage of fibrosis marker α-SMA and I Collagen positively stained cells was estimated and the staining intensity was scored as high (+++), moderate (++), or weak (+; Woodward et al., 2005).
Animal experiments
A total of 15 pregnant female Kunming mice weighing ∼30 g were purchased from the Center of Experimental Animals of Baiqiuen Medical College of Jilin University (Jilin, China). The animals were maintained on a 12 h light/dark cycle with free access to food and water. In the experimental group, 100 μL of TGF-β1 (200 or 400 ng/mL) or PD98059 (125 μM) were injected in the base of the lateral mammary gland. Normal saline infiltration and DMSO was used in the control group. The rats were euthanised 3 weeks following the TGF-β1 injection. Four pairs of mammary glands were dissected and used for the flow cytometry and immunohistochemistry analyses. In addition, we collected cows mammary glands from the two different model diets roughage corn stover (CS) and alfalfa (MF) for immunohistochemistry analyses. All the animal experiments were approved by the local ethics committee, Jilin University, China, and the experimental protocol conformed to the Animal Welfare Act Guide for Use and Care of Laboratory Animals.
Statistical analysis
Experiments presented in the figures are representative of three or more different repetitions. All data are expressed as the mean ± standard error of the mean (SEM) and were analysed with SPSS 13.0 software. A standard Student's t-test was used for the statistical analysis. A value of P < 0.05 was considered statistically significant.
Results
The cultivation and identification of bovine mammary fibroblasts
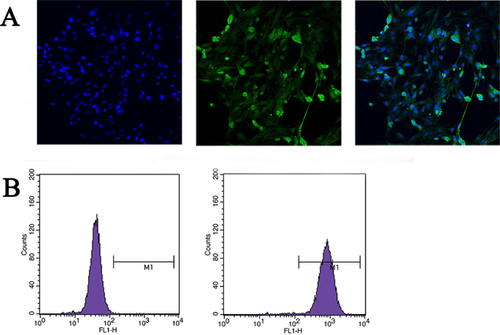
BMFBs were subjected to five rounds of purification and then identified by indirect immunofluorescence and flow cytometry. The results showed that 97.34 ± 2.91% of the cells were vimentin-positive (Figures 1A and 1B), suggesting that the separated cells were fibroblasts and could be used for the subsequent experiments.
TGF-β1 promotes fibroblast proliferation and anti-apoptosis activity
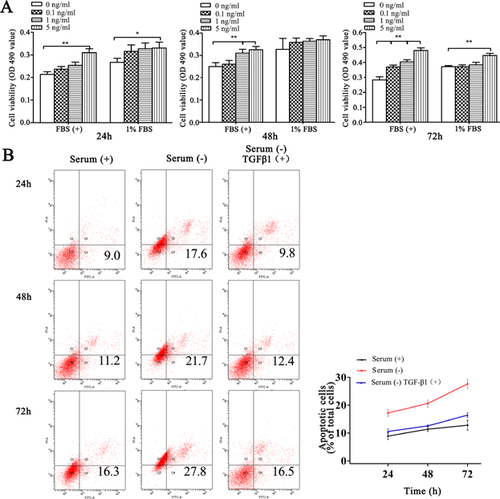
Although many studies demonstrated that TGF-β1 was a pleiotropic cytokine with multiple effects on a number of different cell types and organizational tissues, limited information was available concerning the biological function of TGF-β1 on mammary fibroblasts (Liboi et al., 1988; Chen et al., 2003). First, we used MTT to detect the effect of TGF-β1 on mammary gland fibroblast proliferation. The cells were treated with 0.1, 1 or 5 ng/mL of TGF-β1 for 24, 48 or 72 h. The results showed that treatment with 5 ng/mL TGF-β1 for 24 h in the absence of FBS or presence of 1% FBS significantly promoted fibroblast proliferation (P < 0.05). After 48 h, treatment with 1 and 5 ng/mL of TGF-β1 significantly promoted fibroblast proliferation in the absence of FBS (P < 0.01). TGF-β1 treatment for 72 h in the absence of FBS significantly promoted fibroblast proliferation (P < 0.01) in a dose-dependent manner. In the 1% FBS group, only 5 ng/mL of TGF-β1 significantly promoted fibroblast proliferation (P < 0.01; Figure 2A). Therefore, TGF-β1 promoted fibroblast proliferation in a dose- and time-dependent manner regardless of the presence of FBS. Next, we explored the effect of TGF-β1 on fibroblast apoptosis induced by serum starvation in vitro. The results showed that 48 h of serum starvation significantly increased the apoptosis ratio. Treatment with 5 ng/mL TGF-β1 significantly decreased serum starvation-induced apoptosis; moreover, the anti-apoptotic effect was not significantly different compared with 10% FBS (P > 0.05; Figure 2B). Taken together, these results suggest that TGF-β1 promotes fibroblast proliferation and anti-apoptosis activity.
Activation of the ERK1/2 signalling pathway mediates TGFβ1-induced fibroblast proliferation
Currently, it is clear that TGF-β1 signalling is mediated not only by protein kinase receptor and Smad molecules but also by MAPK (mitogen-activated protein kinase), NF-κB (nuclear factor κB), PI3 K (phosphoinositide 3-kinase)/Akt and other non-Smad signalling pathways (Zhang, 2009). The ERK1/2 signalling pathway is considered the classic MAPK cascade. This pathway consists of MAPKKKs (A-Raf, B-Raf and Raf-1), MAPKKs (MEK1 and MEK2) and MAPKs (ERK1 and ERK2). ERK includes two types of isomers (ERK1 and ERK2) that have 83% homology and molecular weights of 44 and 42 kDa, respectively. These isomers are widely expressed in various tissues. qRT-PCR showed that the ERK1/2 mRNA expression levels after TGF-β1 treatment for 3, 6 and 12 h did not differ compared to the control group (P > 0.05; Figure 3A). However, ERK1/2 mRNA expression was significantly increased after 18 h (P < 0.05). Consistent with the qRT-PCR results, the Western blotting results showed that p-ERK1/2 protein expression was significantly higher than the control at 18 and 24 h (P < 0.01), whereas no difference was detected at the earlier time points.

PD98059 is a flavonoid compound that is widely used as a potent ERK inhibitor (English and Cobb, 2002). Treatment with PD98059 significantly inhibited the fibroblast proliferation induced by TGF-β1 (P < 0.01; Figure 3B), although the p-ERK1/2 protein expression was a little increased at 12 h, it was significantly inhibited at the 18 and 24 h time points (Figure 3C). Moreover, cyclin D1 expression was significantly increased in TGF-β1-treated fibroblasts and could be suppressed by PD98059, suggesting that the fibroblast proliferation induced by TGF-β1 was closely related to the cell cycle. Finally, we examined the cell cycle in TGF-β1-treated fibroblasts. The cell proliferation index (proliferation index, PI) represents the number of proliferating cells in the cell population. The G2 phase is the late stage of DNA synthesis and the M phase is mitosis, which reflects the state of cell proliferation to some extent. Using flow cytometry, we found that TGF-β1 significantly reduced the number of cells in G1 phase (P < 0.05) and increased the number of cells in S phase (P < 0.05) and G2/M phase (P < 0.05) compared to the control group. Additionally, the proliferation index (PI = S + G2/M) was significantly increased (P < 0.05; Figure 3D), suggesting that TGF-β1 significantly accelerated the cell cycle. Treatment with PD95059 significantly reduced the effect of TGF-β1 in the G1 phase and G2/M phase (P < 0.05) and blocked the ability of TGF-β1 to promote DNA synthesis and the cell cycle. Taken together, these results suggest that TGF-β1 activation of the ERK1/2 signalling pathway promotes fibroblast proliferation.
Proliferating effects on fibroblasts were confirmed in mouse mammary glands following long-term continuous injection of TGF-β1
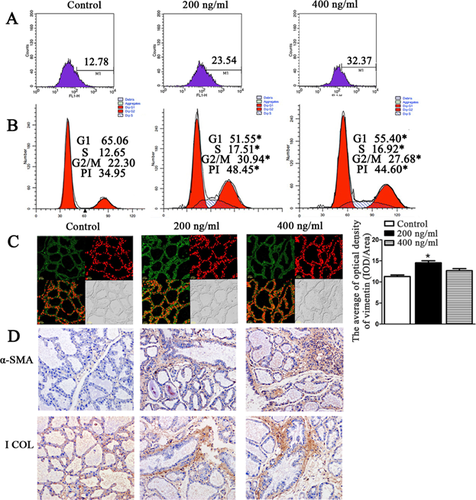
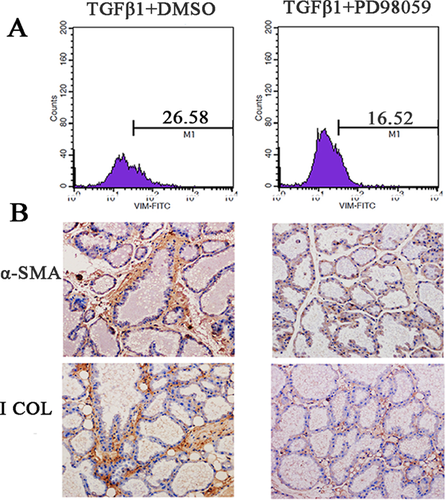
Mouse TGF-β1 is very homologous to bovine TGF-β1. Thus, we explored the effects of TGF-β1 on fibroblast proliferation in mouse mammary glands. As shown in Figure 4A, when we injected 200 and 400 ng/mL of TGF-β1 into mouse mammary glands, the proportion of fibroblasts was increased compared to the control group; the proportion of fibroblasts was highest (32.37%) in the 400 ng/mL group. Moreover, 200 and 400 ng/mL of TGF-β1 not only significantly increased the number of fibroblast cells in the G1, S and G2/M phases but also promoted the cells’ transition from the G1 and S to the G2/M phases, thereby promoting fibroblast DNA synthesis and cell cycle acceleration (Figure 4B). The confocal microscopy results showed that treatment with 200 and 400 ng/mL of TGF-β1 significantly promoted fibroblast proliferation compared to the control group (Figure 4C). Also, we found that 200 and 400 ng/mL of TGF-β1 enhanced the α-SMA and I Collagen expression in mouse gland, which are the fibrosis markers (+++ for injected the TGF-β1, and + for control group; Figure 4D). PD98059 could inhibit the TGF-β1-induced bovine fibroblasts proliferation, so the role of PD98059 against TGF-β1 induction in mouse mammary glands was explored. After PD98059 and TGF-β1 were injected into the mouse, the proportion of fibroblasts and the expression of I Collagen and α-SMA were significantly less than the control group which injected DMSO and TGF-β1 (+++ for injected the PD98059 and TGF-β1; + for control group injected the DMSO and TGF-β1; Figures 5A and 5B).
A straw-based diet induced high levels of TGF-β1 production in bovines that correlated with a proliferating effect on fibroblasts
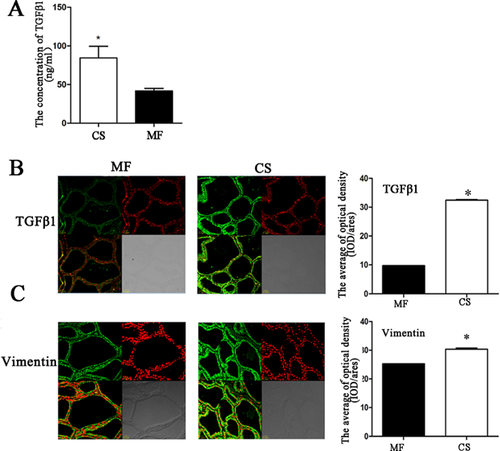
Lactating Holstein cows were used as an experimental model to validate TGF-β1-induced fibroblast proliferation in mammary glands in vivo. Under the two different model diets [roughage corn stover (CS) and alfalfa (MF)] (Zhou et al., 2014), we found that the TGF-β1 level in the blood was significantly increased in the CS group (Figure 6A). In addition, we examined the expression of TGF-β1 and fibroblasts in the mammary tissues of cows from the two groups. The results showed that the expression of TGF-β1 (Figure 6B) and vimentin (Figure 6C) in the mammary glands of the CS group were significantly higher compared to the MF group. This result confirmed that TGF-β1 could promote bovine mammary fibroblast proliferation.
Discussion
This study is the first to confirm that TGF-β1 can promote bovine mammary fibroblast proliferation by the ERK1/2 signalling pathway in vivo and vitro. The results suggest that diet may contribute to the secretion of TGF-β1 and promote fibroblast proliferation, thereby contributing to the development of severe breast fibrosis that leads to the culling of cows and causes serious losses to the dairy industry. Additionally, this study is the first to indicate that nutrition not only affects the lactation of cows but also causes adverse changes in breast structure, thereby providing a theoretical basis for bovine mammary gland development and lactation. These results also provide the foundation for the prevention of bovine mammary fibrosis.
Many studies have shown that TGF-β1 inhibits growth and the induction of apoptosis in mammary epithelial cells in breast tissue while at the same time inhibiting the formation and differentiation of breast ducts and acini (Robinson et al., 1993; Kordon et al., 1995). In 2008, Gal et al. reported that TGF-β1 could induce mammary epithelial cell growth arrest and the occurrence of EMT through the ERK1/2 signalling pathways (Gal et al., 2008). Although studies investigating the biological functions and the mechanism underlying the effect of TGF-β1 on mammary fibroblasts are rare, fibroblasts from other different organizational source tissues have been widely studied. In pulmonary fibrosis, TGF-β1 promotes fibroblast proliferation through the FGF2/ERK pathway, and ERK1/ERK2 protein sustains activity a short time frame increases the capacity of fibroblasts proliferation and promotes the occurrence of EMT (Xiao et al., 2012). Persistent activation of the ERK1/2 pathway was found in the Chinese hamster fibroblast cell line (CC139), leading to cyclin D1 up-regulation and the subsequent acceleration of the cell cycle and promotion of cell proliferation (Squires et al., 2002). In this study, we found that TGF-β1 activated the ERK1/2 signalling pathway and significantly inhibited by the addition of the inhibitor PD98059. Moreover, we found that the PI (PI = S + G2/M) was significantly increased after TGF-β1 treatment. One possible mechanism is the activation of static cells to transition from the G1 phase to S phase and increase DNA synthesis, thereby promoting cell proliferation.
In 1970, Chandler successfully constructed and described the mouse mastitis model in detail (Chandler, 1970), which was considered to be an important tool for research into the pathogenesis and prevention measures for bovine breast disease. Previous studies demonstrated that the TGF-β1 sequence between mature cows and mice contained a difference of only one amino acid; moreover, both precursors contained the Arg-Gly-Asp-Leu sequence (Sporn et al., 1986). Due to its high homology and ease of obtainment, the effects of TGF-β1 on mammary gland development, fibroblast proliferation and the cell cycle were tested in the mouse model. Additional, TGF-β1 is a potent mediator of fibrosis and causes an accumulation of ECM proteins in many different cells. (Lasky and Brody, 2000; Woodward et al., 2005). This study found that injection of mouse with TGF-β1 resulted in significant fibroblast proliferation, the promotion of the transition of the cells from the G1 to S and G2/M phases and an increase in DNA synthesis. The confocal microscopy results also confirmed that TGF-β1 promoted fibroblast proliferation and enhanced the fibrosis markers α-SMA and I Collagen expression in stromal of the mouse mammary gland. In addition, PD98059 blocked the effects of long-term high levels TGF-β1. These results also indicated that the TGF-β1 through the ERK 1/2 signalling pathway mediate the fibroblasts proliferation and the fibrosis progress.
Recently, consumer demand for dairy products greatly increased in China. However, milk production is lower in northern China compared to southern China. A survey found that dairy farming in northern China used roughage that had a lower nutritional value than corn stover, whereas high-quality alfalfa was more common in southern China due to differences in the geographical environment. Under the two different model diets, we found that cows fed corn stover (CS) as roughage exhibited significantly increased the TGF-β1 level in the blood and in mammary gland compared to cows fed alfalfa (MF). In addition, vimentin expression in the CS group tended to be increased compared with the MF group, confirming that TGF-β1 could promote bovine mammary gland fibroblast proliferation. These results also indicated that the nutritional levels had an impact on the body's endocrine levels and growth.
Conclusions
Our data reveal that TGF-β1 significantly promoted the proliferation of BMFBs through the ERK1/2 signalling pathway. This study verified the role of TGF-β1 in the mammary glands of mice and cows. The dairy breeding experiments also suggest that different feeds may increase the secretion of TGF-β1, thereby promoting fibroblast proliferation and leading to adverse changes in the structure of the mammary gland that decrease the cows’ milk yields and protein rates. This study helps elucidate the biological functions of TGF-β1 in mammary gland development.
Acknowledgements and funding
We gratefully thank the teachers and students of the “Dairy 973” team for their assistance in conducting this experiment. We also acknowledge the electron microscope room of the School of Basic Medical Science of Jilin University for their help on our experiments. This work is supported by National Basic Research Program of China (973 Program), the grant number: 2011CB100805.
Conflict of interest
No authors in this manuscript declare any conflict of interest.




