Isolation of lung multipotent stem cells using a novel microfluidic magnetic activated cell sorting system
Abstract
In recent years, more and more research has shown that the lung is an organ of regenerative potential, with several types of stem/progenitor cells undergoing proliferation and differentiation after lung injury and participating the injury repair process. Mouse lung multipotent stem cells (MLSCs) have extensive self-renewal ability in culture and could differentiate into endothelial and lung epithelial (alveolar epithelial type 1, 2, and Clara) cells in vitro. But the research of MLSCs was limited due to its rarity. In this study, we introduced a novel microfluidic magnetic activated cell sorting system in the isolation of MLSCs. The sorted MLSCs had better viability and purity. They were identified by colony formation efficiency and differentiation ability and they have self-renewal and differentiation capacities, highlighting their stem cell properties.
Abbreviations
-
- BASC
-
- bronchoalveolar stem cells
-
- CCSP
-
- clara cell secretary protein
-
- CFE
-
- colony formation efficiency
-
- DMEM
-
- high glucose Dulbecco's modified Eagle's medium
-
- FACS
-
- fluorescence activated cell sorting
-
- LIF
-
- leukemia inhibitory factor
-
- MACS
-
- magnetic activated cell sorting
-
- MLSCs
-
- mouse lung multipotent stem cells
-
- pro-SPC
-
- pro surfactant protein C
Introduction
Lung stem and progenitor cells have been reported to have different functions in lung homeostasis; they could migrate, proliferate, and differentiate to terminally differentiated cells and may play an important role in bronchiolar and alveolar epithelial cells injury repair (Kim et al., 2005; Hegab et al., 2010; McQualter et al., 2010; Chapman et al., 2011). Bronchoalveolar stem cells (BASC) were recently identified in adult mouse lung as a rare cell population located in the bronchoalveolar duct junction. BASCs have the properties of stem cells and are linked to lung adenocarcinoma initiation (Kim et al., 2005; Ventura et al., 2007). They were identified and purified by fluorescence activated cell sorting (FACS) based on being positive for Sca-1 and CD34 and negative for CD45 and CD31. This population was also characterized by its double positive staining characteristics for both alveolar epithelial type 2 (AEC2) cell marker, pro-surfactant protein C (pro-SPC) and Clara cell marker, Clara cell secretary protein (CCSP). BASCs were maintained through multiple passages in vitro and they may play an important role in lung tumorigenesis (Kim et al., 2005; Noh et al., 2009). Hegab et al. (2010) identified a set of Sca-1-pos, CD45-neg, and CD31-neg MLSCs that were self renewing, clonogenic and multipotent in vitro and that could differentiate into endothelial and lung epithelial (alveolar epithelial type 1, 2, and Clara) cells in vitro. Transfer of these cells into mouse models of lung injury significantly improved survival and minimized lung destruction. These cells may provide useful tools for the study of lung stem cells and the assessment of new therapeutic approaches for lung diseases. BASCs and lung multipotent stem cells are very rare in mouse lung. The proportion of BASCs is about 0.008–0.064% (Kim et al., 2005) and MLSCs is about 1.4% in whole lung single-cell suspension (Hegab et al., 2010), so the isolation remains a problem.
Many technologies have been developed for the lung stem cell isolation such as fluorescence activated cell sorting and magnetic activated cell sorting (MACS). The most widely used cell separation technology is FACS. It allows for the isolation of cell subsets with high purity based on fluorescent light-scattering properties originating from antibodies that are bound on the cell surface. However, FACS is very expensive and requires highly trained operators; also, FACS has limitations in terms of the low viability of the sorted cells and its inability to process large number of cells (Wognum et al., 2003; Cammareri et al., 2008). The MACS allows cells to be separated by incubating with magnetic microbeads coated with antibodies against a particular surface antigen. Afterwards the cell solution is transferred on a column placed in a strong magnetic field. In this step, microbeads labeled cells stay on the column, while other cells flow through. With this method, the cells can be separated positively or negatively with respect to the particular antigens. The advantage of MACS is to isolate the desired cells efficiently from a large number of cells within a short period of time. However, relatively low purity is its main limitation (Geens et al., 2007).
The IsoFlux System (Fluxion Biosciences, Inc., South San Francisco, CA) was designed to address these limitations by providing high-sensitivity rare cell isolation coupled with a novel cell retrieval mechanism. The system uses immunomagnetic beads that facilitate the use of single or multiple capture antibodies to target cells. The initial purpose of IsoFlux System is to isolate rare circulating tumor cells (CTCs) from peripheral blood efficiently (Zhang and Austin, 2012; Harb et al., 2013); here, we introduce a method using IsoFlux System to isolate MLSCs for the first time. The sample passes through a microfluidic device that contains an isolation zone to capture target cells on the upper surface of the cartridge in an externally applied magnetic field. Fluid flow was controlled pneumatically from an externally supplied pressure inside the instrument. A diaphragm pump and electropneumatic regulator create a controlled operating pressure of 2 psi. This pressure gets distributed to the sample reservoirs to initiate flow through the microfluidic channel. The roof of the microfluidic channel decouples from the rest of the cartridge and transfers off-chip with the target cells still retained on its surface, providing near perfect transfer efficiency (Harb et al., 2013). This microfluidic magnetic cell sorting system provides a new way in the isolation of rare cells.
Materials and methods
Single-cell suspension preparation
Animals used in this study were C57BL/6 mice, 4–6 weeks old, maintained in pathogen-free conditions with the approval of the Ethical and Protocol Review Committee of the Third Military Medical University. Single-cell suspensions were prepared from two mice each time. In brief, anesthetize mouse with 45 mg/kg pentobarbital, cut into ribcage, perfuse 10 mL PBS through right ventricle until lungs were cleared of blood. Then, dissect out the lungs. Subsequently, 2 mL dispase I (10 U/mL; BD Pharmingen, San Diego, CA) was injected through the trachea. The lungs were incubated in a 37°C shaking incubator for 30 min in 10 mL of dispase (10 U/mL), 0.001% DNAse (Sigma, St. Louis, MO), and 2 µg/mL collagenase/dispase (Roche Diagnostics, Indianapolis, IN). The lobs of lungs were minced and incubated for 2 min. This suspension was filtered on 100 and 40 μm filters and centrifuged (Kim et al., 2005).
Lung multipotent stem cells isolation by IsoFlux™
The CD45-pos cells (hematopoietic cells) and CD31-pos cells (endothelial cells) are magnetically labeled with anti-murine CD45 antibody-coated and anti-murine CD31 antibody-coated microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Then, the single-cell suspension is loaded onto the Sample Inlet of the cartridge, which is then placed in the sample tray of IsoFlux™ (Figure 1). The sample flows into isolation zone at low velocities and sees an externally applied magnetic field from above the collection substrate. CD45-pos and CD31-pos cells with magnetic beads are attracted to the roof of the isolation zone, whereas other unlabeled cells are biased from flow and gravitational forces to continue down the channel and into the unlabeled cell zone (Figure 1).
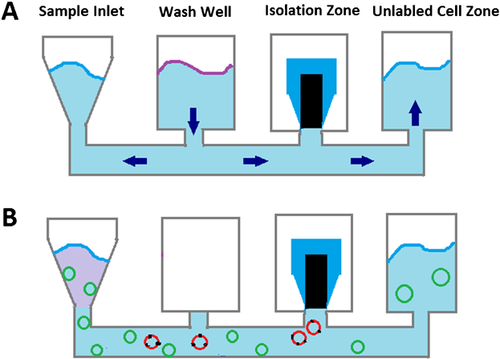
CD45-neg and CD31-neg cells were then incubated with anti-mouse Sca-1 antibody conjugated to FITC (Miltenyi Biotec), after which magnetic labeling of Sca-1-pos cells can be achieved using Anti-FITC MicroBeads. Then, cells were flowed through the channel at a flow rate of 20 µL/min by applying a head pressure of 2 psi to the inlet well. Cells flow into isolation zone and Sca-1-pos cells with magnetic beads are attracted to the roof of the isolation zone. The roof of the isolation zone is a polymeric disk that is separate from the construction of the main flow channel. After processing, the disk containing the cells in a hanging drop is inserted into a tube (Figure 1).
Cell culture
The isolated CD45-neg CD31-neg Sca-1-pos cells were dispensed into a 6-well plate for culture. The seeding density is 500 cells/cm2. The culture medium contains high-glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS, 10 ng/mL leukemia inhibitory factor (LIF, SinoBio, Shanghai, China), glutamine, penicillin/streptomycin, and 1% nonessential amino acids.
Colony formation assay
To determine the colony formation effiency (CFE) of sorted populations, the CD45-neg CD31-neg Sca-1-pos cells and CD45-neg CD31-neg Sca-1-neg cells were seeded in 6-well plate at a density of 500 cells/cm2. After culturing for 7 days, these cells were fixed with 4% paraformaldehyde, washed in PBS, and stained with 1% Rhodamine B. Colonies were then counted under a microscope. Only colonies >32 cells were scored (Tudor et al., 2007). The CFE was designated as the number of colonies divided by the total number of seeded cells.
Lung multipotent stem cell differentiation induced by bronchoalveolar lavage from LPS-induced lung injury mice
LPS-induced lung injury was induced in mice (6–8 weeks) by instillation of bacterial LPS, 4.5 mg/kg, intratracheally in 200 μL PBS. LPS was injected into the trachea followed by an injection of 0.6 mL of air to ensure adequate dispersion of the injected material into the distal lung. Mice were sacrificed; bronchoalveolar lavage (BAL) and lung extract were prepared at 72 h. Mice lungs were lavaged to capacity six times with HEPES-buffered DMEM. The BAL was centrifuged to remove cells, aliquoted and frozen at −80°C. Minced, unfiltered, dispase digested lung was incubated in HEPES-buffered DMEM at 37°C, with gentle agitation, for 3 h (1 minced lung/10 mL DMEM), filtered through 40 μm filter, centrifuged to remove cells, aliquoted, and frozen at −80°C (Buckley et al., 2011).
Sorted MLSCs were resuspended in 100 μL of Matrigel (BD Biosciences) prediluted 1:1 (vol/vol) with media were added to a 24-well transwell filter insert (Millicell-CM; Millipore) in a 24-well tissue culture plate containing 400 μL of media (McQualter et al., 2010). Differentiation medium contains 100 ng/mL bronchoalveolar lavage from LPS-induced lung injury mice, high-glucose DMEM supplemented with 10% FBS, glutamine, penicillin/streptomycin, and 1% nonessential amino acids. Differentiated colonies were dissociated from the Matrigel after 10–15 days and immunofluorescently labeled for aquaporin-5 (AQP5), a mark protein of alveolar type 1 epithelial cell (AT1) (Desai et al., 2014).
Results
Isolation and culture of lung multipotent stem cells
We usually retrieve 1.2–2.0 × 107 total cells and 2.6–4.1 × 104 MLSCs per mouse lung. The sorted MLSCs were labeled with FITC conjugated Sca-1 antibody, so we verified the cell purity by the FITC fluorescence. A total of 96–99% cells in the isolation zone were labeled with FITC fluorescence (Figure 2A), whereas no cells were labeled in the unlabeled cell zone (Figure 2B).

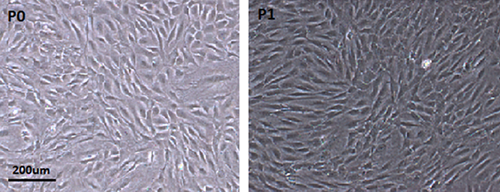
Cells were seeded at a density of 500 cells/cm2. After culturing for 3 days, the number of adherent cells increased and formed into 5–8 cells’ colonies. After culturing for 7 days, spindle-shaped cell colonies gradually increased in number and size as observed by the inverted phase contrast microscope (Figure 3).
Colony formation efficiency
The CD45-neg CD31-neg Sca-1-pos cells had a CFE of 38.6 ± 6.9%, but the CD45-neg CD31-neg Sca-1-neg cells had a much lower CFE, 5.9 ± 1.2%. This observation indicated that lung multipotent stem cells exhibited extensive self-renewal in culture; they have a higher proliferative potential than CD45-neg CD31-neg Sca-1-neg cells.
Differentiation induced by the milieu of acute lung injury
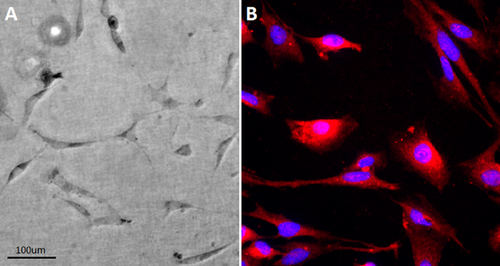
After culturing with bronchoalveolar lavage from LPS-induced lung injury mice for 10–15 days, the multipotent stem cell colonies differentiated to flat, long, and thin cells, they showed a complex structure that resembled a alveolar-like shapes (Figure 4A). These cells strongly expressed AT1 cells marker-AQP5 (Figure 4B).
Discussion
In the context of lung regeneration, the main purpose of the multipotent lung stem cells is to generate new cells and maintain structure (Gadepalli et al., 2013). Scientists have characterized multipotent stem cells from different tissues and have inferred that different organs use different strategies to renew themselves (Rawlins and Hogan, 2006). There is growing evidence of resident MLSCs in murine lung poss self-renewal and different differentiation capacities, highlighting their stem cell properties (Kim et al., 2005; Hegab et al., 2010). MLSCs can give rise to endothelial and lung epithelial cells in vitro and that cell therapy with MLSCs can improve survival and minimize lung damage after injury (Hegab et al., 2010).
However, the methods of isolating MLSCs are limited because the cell population is very small (Kim et al., 2005; Hegab et al., 2010). In order to improve the sorting technique, we developed a sorting assay of MLSCs using IsoFlux system, which combined the advantages of FACS and MACS. Using this technique, MLSCs were sorted in a two step assay. First, CD45-neg and CD31-neg cells were collected by the removal CD45-pos and CD31-pos cells. Then, Sca-1-pos MLSCs were sorted. The in vitro cultured MLSCs had a significantly higher CFE than CD45-neg CD31-neg Sca-1-neg cells, indicating that they exhibited extensive self-renewal ability in culture and they had a higher proliferative potential. The differentiation potential of adult stem cells was usually associated with their germ layer of origin. MLSCs showed great plasticity beyond the lineages; they had the ability to differentiate into mesenchymal cells (Hegab et al., 2010) and our study showed that they can also differentiate into AT1 by culturing with bronchoalveolar lavage from LPS-induced lung injury mice.
The IsoFlux System was designed to address these limitations by providing high-sensitivity rare cell isolation coupled with a novel cell retrieval mechanism. The fluid flow was controlled pneumatically from an externally supplied pressure inside the instrument. A diaphragm pump and electropneumatic regulator create a controlled operating pressure of 2 psi. This pressure gets distributed to the sample reservoirs to initiate flow through the microfluidic channel. The flow is used to direct cells across a high magnetic field isolation zone at a relatively low velocity (Harb et al., 2013). This controls both the magnetic force applied to each bead and the cell's residence time in the isolation zone and results in improved capture efficiency. The low velocity of flow makes sure that the viability of the sorted cells are much higher than FACS. It has relatively higher purity than MACS, the microfluidic approach leads to reduced background contamination (Harb et al., 2013). It provides an effective method in the study of MLSCs and other rare cells.
Conclusions
The novel microfluidic magnetic activated cell sorting system provides a new method in the isolation of MLSCs. The sorted MLSCs have better viability and purity. They have possessed self-renewal and differentiation capacities.
Acknowledgement and funding
This work was supported by Major State Basic Research Development Program of China (2012CB518104).




