Hepcidin and its potential clinical utility
Abstract
A number of pathophysiological conditions are related to iron metabolism disturbances. Some of them are well known, others are newly discovered or special. Hepcidin is a newly identified iron metabolism regulating hormone, which could be a promising biomarker for many disorders. In this review, we provide background information about mammalian iron metabolism, cellular iron trafficking, and the regulation of expression of hepcidin. Beside these molecular biological processes, we summarize the methods that have been used to determine blood and urine hepcidin levels and present those pathological conditions (cancer, inflammation, neurological disorders) when hepcidin measurement may have clinical relevance.
Abbreviations
-
- AA
-
- amino acid
-
- A1AT
-
- alpha1 antitrypsine
-
- A2MG
-
- alpha2 macroglobuline
-
- BBB
-
- blood brain barrier
-
- BMCEs
-
- brain microvascular endothelial cells
-
- BMP
-
- bone morphogenetic protein
-
- CNS
-
- central nervous system
-
- DMT1
-
- divalent metal trasporter1
-
- ER
-
- endoplasmic reticulum
-
- HAMP
-
- hepcidin antimicrobila peptide
-
- HFE protein
-
- human hemochromatosis protein
-
- IL-6
-
- interleukin-6
-
- IRP/IRE
-
- iron-regulatory protein/iron-responsive element
-
- JAK/STAT
-
- Janus kinase/signal transducers and activators of transcription
-
- MS
-
- mass spectrometry
-
- sTfR
-
- soluble transefrrine receptor
-
- TfR
-
- transferrin receptor
Introduction
Iron is one of the essential transition metals not just for humans but practically for all living organisms. However, iron-ion in its free form is potentially toxic, may damage cellular macromolecules leading to tissue injury and disease. To minimize the risk of toxicity, living organisms developed potent biological chelators that maintain the availability of iron while transport is safe within and outside of the cell (Jomova and Valko, 2011). Iron is found in the body both in a functional form and as storage iron (Pantopoulos et al., 2012). About 68% of the total body iron is bound to hemoproteins and non-heme proteins and ca. 30% may be found in the complex form of the iron storage proteins ferritin and hemosiderin (Ganz, 2013). Only a small fraction of iron, the transferrin bound serum iron is measurable in the serum (Gkouvatsos et al., 2012). Unfortunately, the information value of serum iron level to assess the organism's overall iron status is limited (Cook, 1999).
Over the years, several diagnostic markers have been implemented to assess the iron status, including transferrin level, transferrin saturation, ferritin level, and soluble transferrin receptor. Albeit these laboratory parameters substantially advanced the differential diagnosis of disorders involving iron metabolism, there is still room to improve the diagnostic arsenal. Iron metabolism is a complex system still not known in every detail, and it is also influencing and influenced by many physiological and patho-physiological processes such as inflammation, malignancy, or even neurologic disorders (Camaschella, 2013). The presence or the co-existence of these conditions significantly lessens the specificity and usefulness of the presently available iron status biomarkers.
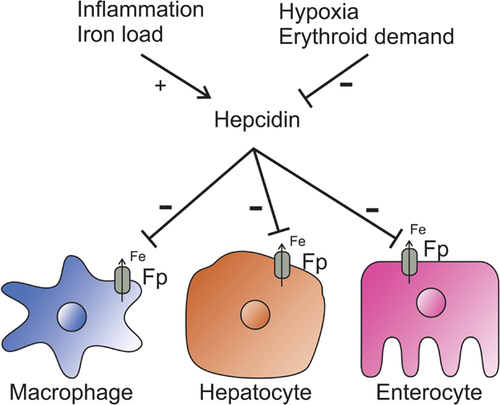
Hepcidin is a recently discovered peptide hormone, which is (yet) the only known regulatory hormone of the iron homeostasis (Lesbordes-Brion et al., 2006). Hepcidin regulates iron transport by binding to the iron transporter ferroportin and upon binding, initiates the internalization and degradation of ferroportin. This process eventually leads to the decrease of available circulating iron levels (Figure 1). Revealing the regulation of hepcidin expression provides at least two benefits: (1) it improves our understanding of the regulation of iron metabolism and (2) we can use the determination of the circulating hepcidin level as a differential diagnostic method. Since its discovery, hepcidin was proposed both as a diagnostic and as a therapeutic tool (Ruchala and Nemeth, 2014). In this review, we summarize the recent findings about hepcidin's synthesis and effect on the iron metabolism and we also discuss its potential value as a biomarker in the differential diagnosis in anemia, inflammation, and other disorders that are related to iron metabolism.
Synthesis and the effect of hepcidin
Synthesis
The peptide hormone hepcidin is the principal regulator of systemic iron homeostasis. Hepcidin was first isolated from urine during a screen for antibacterial peptides (Park et al., 2001). Hepcidin is similar in nature to defensins; its name reflects the proposed antibacterial activity and the place of origin since hepcidin is mainly produced by the liver. Soon after its identification, the role of hepcidin as the major (and so far only known) hormonal regulator of iron metabolism was discovered in transgenic mice (Nicolas et al., 2001; Pigeon et al., 2001).
Hepcidin is expressed from the HAMP gene as an 84 amino acid preprohormone. Beside the liver, the expression of hepcidin has been confirmed in a number of tissues of various vertebrates including humans: relatively high level of hepcidin is found in the heart, kidneys, pancreas, and adipose tissue. The secretion of hepcidin from these organs may also contribute to the regulation of the iron in the circulation, which could explain the elevated level of hepcidin often seen in obesity associated anemia (del Giudice et al., 2009). In other cells types (salivary glands, cells of the central nervous system, leukocytes, and macrophages), the expression level of hepcidin is also present but at a much lower rate. It was proposed that in these cells, hepcidin has an autocrine/paracrine function and this type of action has already been demonstrated experimentally in macrophages (Theurl et al., 2008).
The 84 amino acid preprohepcidin is processed in the ER and Golgi. After the cleavage of the ER-leading sequence from its N-terminus, the 60 amino acid prohepcidin is further cleaved by furin and other proportion convertases forming the mature, active 25 amino acid peptide hormone hepcidin (Valore and Ganz, 2008). The structure of hepcidin is unique as out of the 25 amino acids 8 are cysteines, forming four disulphide bridges (Hunter et al., 2002). For years, it was declared that hepcidin contains a vicinal disulphide bond, but now, it is evident that the hairpin structure of the molecule has been kept by a different arrangement of the disulphide bridges (Jordan et al., 2009).
Both prohepcidin and the mature hepcidin can be found in the cells and in the bloodstream as well. In the latter, shorter forms of 20, 22, and a lately described 24 (Laarakkers et al., 2013) amino acid long hepcidin can also be detected, although their physiological roles are not clear (Campostrini et al., 2010). It is proposed that mature hepcidin and the naturally occurring N-terminal truncated shorter forms both play a role in host defense mechanism against microbial invasion but in different ways. Supposedly, the full length hormone's function is to reduce the available iron for microbes while the 20 AA peptide has been found to bind to bacterial DNA (Brancatisano et al., 2014). This latter mechanism is unusual considering that hepcidin with its four disulphide bridges has a very similar structure to defensins, which are known to carry out their antibacterial effect by forming pores in the bacterial membranes (Hocquellet et al., 2012). Interestingly, the scientific interest for the antimicrobial effect of hepcidin has declined in recent times, although some newer studies also confirmed this effect in various other animals (fishes, crocodile) (Hao et al., 2012; Tao et al., 2014b). Another recent development in this field is the successful use of a synthetic 20 amino acid peptide analogous to hepcidin in antifungal treatment resistant local inflammations (Del Gaudio et al., 2013).
Regulation of hepcidin expression
A large number of excellent, detailed reviews deal with the intracellular signalling pathways of the regulation of hepcidin expression (Gaffney-Stomberg and McClung, 2012; Finberg, 2013; Zhao et al., 2013). Hepcidin expression is influenced by several factors including (1) iron level of the blood and iron store of the body, (2) the activity of erythropoiesis, and (3) inflammatory mechanisms (Ganz, 2011; van Eijk et al., 2011). Circulating transferrin receptor2 (Trf2), HFE, bone morphogenetic protein 6 (BMP6), and hemojuvelin are the most important components of extracellular iron sensing (Huang et al., 2005; Andriopoulos et al., 2009; Worthen and Enns, 2014). BMP6 and hemojuvelin activate the BMP receptor/SMAD pathway, which will result in induction of hepcidin expression (Zhao et al., 2013). Transferrin binds to transferrin receptor 2 (TfR2) and, according to a recent study, Trf2, along with HFE, also converges into the BMP receptor/SMAD pathway (Wu et al., 2014). Others claim that it is possible to affect hepcidin expression with Tf-bound iron without changing the expression of BMP6 (Zhang et al., 2014). In this mouse model, the results prove that the main regulator of hepcidin expression was the high iron demand of the body.
A new study showed that there are two BMP-responsive elements in hepcidin promoter (Casanovas et al., 2014). Matriptase-2, a membrane bound serine protease of hepatocytes, is a negative regulator of hepcidin expression (Wang et al., 2014), probably by releasing hemojuvelin from the membrane, thus inhibiting the activity of BMP6. Interestingly furin, which is involved in hepcidin maturation, is another enzyme that solubilizes hemojuvelin. Expression of furin is regulated by several factors including hypoxia (Poli et al., 2010). This mechanism might explain how hypoxia affects hepcidin expression.
Serum iron level is not the only factor having an effect on the regulation of hepcidin expression. The erythropoietic activity (Kato, 2010) or an occasional hypoxia (Piperno et al., 2011) may overrule the signal of the iron supply. Erythropoietin has an inhibitory effect on hepcidin expression (Pinto et al., 2008; Gammella et al., 2015). The delay between erythropoietin level increase and hepcidin expression suppression suggests the involvement of special factor(s) (Ravasi et al., 2014). The newly discovered hormone, erythroferrone, might be the mediator of erythropoietin action in the negative regulation of hepcidin during increased erythropoiesis (Kautz and Nemeth, 2014). Regulation of hepcidin expression is definitely more complex than it was thought. The strength of the various signals has a synergistic effect on each other. In cultured hepatocytes, the missing signals from other tissues might explain the surprising results that hepcidin expression actually decreased after iron treatment.
The third most potent hepcidin expression regulator is interleukin-6 (IL-6), which acts through the activation of the JAK/STAT3 intracellular signalling pathway. IL-6 and some other interleukins are elevated during infections and chronic inflammatory diseases (Nemeth et al., 2004; Kartikasari et al., 2008; Zhang and Rovin, 2010), and all these pathological situations were accompanied with increased hepcidin expression.
Beside the above well characterized regulators, additional factors were proposed that might impact hepcidin expression, such as vitamin D, oestrogen, or testosterone (Bacchetta et al., 2014; Qian et al., 2015) but their exact functions are not determined yet. Special but not a unique control of hepcidin (HAMP) gene expression is that prohepcidin inhibits its own transcription by binding to the promoter of its gene at the STAT3 regulatory site. The fact that only the free and not the A1AT-bound prohepcidin has autoregulatory effect raises the possibility of a dual regulatory function of A1AT-prohepcidin interaction (Pandur et al., 2013)
Regulation of iron metabolism
The regulation of iron metabolism may be divided into two levels: systemic (distribution of iron among the various tissues of the body) and intracellular. Iron entering a cell is either used for biosynthetic reactions or the unused iron is stored in the cytoplasm or in the mitochondria in the form of ferritin or mitoferrin, or can leave the cell (Hentze et al., 2010). The iron metabolism of the body is orchestrated by hepcidin, the only known iron regulatory hormone (Park et al., 2001). Mammals can regulate iron at the entry level (absorption/uptake) but there is no known regulation at the exit level (via excretion). Natural iron loss occurs only through bleeding and the decomposition of dying cells. Therefore, the regulation of iron homeostasis depends primarily on the physiological uptake and cellular entry mechanisms (Zhang and Enns, 2009).
The dietary iron is absorbed mostly by enterocytes in the duodenum in a reduced, ferrous (FeII) form, or in a smaller part in the form of heme or ferritin. Ferrous iron is transported into enterocytes via DMT1 (divalent metal transporter 1), while heme is taken up by special heme transporters (Nam et al., 2013). Both the transported FeII and the iron released from heme by the action of heme oxygenase are leaving the enterocytes by the action of the solely iron exporter ferroportin (Ganz, 2005). Excess iron may be stored within enterocytes.
The transported iron is oxidized by haephestin into FeIII ion, which can be taken up by transferrin in the blood and is carried to every tissue for utilization. The other major source of blood iron is macrophage cells where the degradation of senescent red blood cells occurs (Munoz et al., 2009). Macrophages have high activity of hemoxygenase that degrades heme and releases iron for recycling.
Iron, taken up from the bloodstream by cells will be incorporated into functional heme (ferrochelatase) and non-heme proteins. The tissues requiring most of the iron are the bone marrow (haemoglobin), heart, and striated muscle (myoglobin), liver, and the central nervous system (cytochromes). Intracellularly, mitochondria play an indispensable role in the biosynthesis of both heme and iron-sulphur clusters (Richardson et al., 2010; Paul and Lill, 2014).
Excess iron, which is not used for synthesis (frequently due to mitochondrial disturbances) is stored in the form of ferritin bound iron (Garrick and Garrick, 2009). Ferritin may be located in the cytoplasm as well as in the mitochondria. This molecular form is able to bind in excess of four thousand ferric ions; thus, it may act as an effective iron buffer within the cell. Ferritin is present in the serum in small amounts and is a good indicator of the amount of iron stores in the body. However, ferritin is also an acute phase protein, which makes differential diagnosis complicated (Knovich et al., 2009) (Table 1).
| Name | Gene symbol | Role in iron metabolism | Cellular localization |
|---|---|---|---|
| Divalent metal transporter 1 | DMT1 | Ferrous iron transporter | Apical membrane of enterocytes, membrane of lysosomes |
| Coeruloplasmin | CP | Ferroxidase | Blood plasma |
| Haephestin | HEPH | Ferroxidase | Apical membrane of enterocytes |
| Transferrin | TF | Iron transport | Blood plasma |
| Transferrin receptor | TfRC | Cellular iron uptake | Cell membrane |
| Ferroportin-1 | SLC40A1 | Cellular iron export | Basolateral membrane of enterocytes, hepatocytes, macrophages |
| Ferritin | FTL and FTH1 | Iron storage | Reticuloendothelial system |
| Mitochondrial ferritin | FTMT | Ferroxidase | Mitochondrion |
| Mitoferrin | SLC25A37 | Mitochondrial iron transporter | Mitochondrion inner membrane |
| Ferrochelatase | FECH | Heme biosynthesis | Mitochondrion |
| Heme oxygenase 1 | HMOX1, | Heme catabolism | Cytoplasm |
| Frataxin | FXN, | Iron-sulphur complex assembly | Mitochondrion |
| Heme carrier protein 1 | SLC46A1, | Folate and heme transport | Cell membrane |
| Hemojuvelin | HJV | Regulation of hepcidin expression | Cell membrane, extracellular fluid |
| Transferrin receptor 2 | TfR2 | Transferrin bound iron uptake | Cell membrane |
| Bone morphogenetic protein 6 | BMP6 | Regulation of hepcidin expression | Secreted |
| Interleukin 6 | IL6 | Induction of hepcidin expression | Blood plasma |
| Human hemochromatosis protein | HFE | Regulation the interaction of the transferrin receptor with transferrin | Cell membrane |
| Erythroferrone | ERFE | Suppression of hepcidin expression | Blood plasma |
| Mobilferrin | Cytoplasmic iron transport | Transferrin containing vesicles, cytoplasm |
The effect of hepcidin
Serum hepcidin decreases the level of circulating iron by binding to its receptor, ferroportin, an iron exporter located in the membranes of hepatocytes, macrophages, enterocytes, and placental cells (Nemeth et al., 2006). The N-terminal amino acids of hepcidin are necessary for ferroportin binding (Nemeth et al., 2006). This hepcidin–ferroportin interaction activates a signaling pathway (De Domenico et al., 2011b), which triggers the internalization, ubiquitination, and degradation of the ferroportin–hepcidin complex (Ramey et al., 2010; De Domenico et al., 2011a). This complex regulatory mechanism will result in the reduction of blood iron level and the intracellular sequestration of iron (Ganz and Nemeth, 2012). It is worth to mention that ferroportin expression is highly regulated by transcriptional factors, the availability of heme, iron, and some other metals (zinc and copper), and posttranscriptionally via the well-defined IRP/IRE system (Beaumont, 2010). New discovery on this field demonstrated that certain inflammatory pathways mediated by toll-like receptors can alter ferroportin expression without effecting hepcidin level (Guida et al., 2015). This rapid change in ferroportin expression occurs in macrophages, liver, and spleen cells, which are the major sources of recycled iron in the body.
As many other hormones in the blood hepcidin binds to a carrier protein, alpha-2-macroglobulin (A2MG) (Peslova et al., 2009), this interaction enhances the biological effect of hepcidin and slows down the elimination of the hormone via the kidneys. The A2MG-hepcidin interaction is also part of the inflammation reaction (at low pH hepcidin dissociates from A2MG) (Huang et al., 2013). Interestingly, prohepcidin can also bind to alpha1 antitrypsin (A1AT). The importance of this phenomenon is not known yet but it may regulate the final step of hepcidin maturation, providing a pool of rapidly available hormone when required (Pandur et al., 2009).
Current analytical tools to measure hepcidin in human samples and diagnostic considerations
Clarifying the link between iron homeostasis and hepcidin expression opened up the possibility that hepcidin measurements might provide benefit to clinical laboratories (Nicolas et al., 2002). In the blood, the bioactive hepcidin is 25 amino acid long, but two shorter hepcidin variants—hepcidin-22 and 20—are also present (Kemna et al., 2007) among significant amount of prohepcidin. All these forms of hepcidin have been also found in the urine.
For a relatively long period of time, diagnostic laboratory scientists relied on ELISA-based prohepcidin measurements rather than on the direct measurement of hepcidin. Due to the lack of appropriate specific antibody for hepcidin (the short peptide contains only a few epitopes, and its high degree of conservation causes inappropriate immune response in host animals), the measurement of the longer prohepcidin form was more reliable. Unfortunately, results failed to give correlation between prohepcidin and hepcidin or other iron homeostasis parameters and/or the hematology status in patients (Kemna et al., 2005; Nagy et al., 2010; Vermeulen and Vermeersch, 2012). However, some authors found correlation between the iron homeostasis status and prohepcidin levels in inflammatory bowel diseases (Oustamanolakis et al., 2011), while others found that prohepcidin may be a negative acute phase product following cardiac surgery (Maruna et al., 2009). Nevertheless, with the progress of more reliable techniques, predictably direct hepcidin measurement will be in the centre of interest for laboratory medicine (Kroot et al., 2011; Zipperer et al., 2013).
Hepcidin measurements include several methods; the most prominent ones are RIA, ELISA, and mass spectrometry (MS) (Macdougall et al., 2010; Goyal et al., 2013). These methods improved significantly both in their specificity and sensitivity during the past 5 years. Nonetheless, there is no consensus method yet and these various techniques yield diverse results both in quantitative and qualitative terms (Kroot et al., 2012). Therefore, the reference ranges of the applied procedures are not comparable and the reproducibility of the results is sometimes questionable. In the absence of reference methods and standardized reference materials, alternate ways are recommended to achieve the required precision, sensitivity, and specificity criteria of the clinical laboratories' quality control assurance (Kroot et al., 2012). This necessity demands the use of methods with the lowest analytical errors, the greatest dynamic range and universal “standard” samples that enables comparison of results between laboratories. Briefly, further analytical improvements are needed before hepcidin measurements can occupy their place in the main line of laboratory methods.
Despite these handicaps, the efforts related to explore the place of hepcidin among the known laboratory parameters yielded significant discoveries about circulating hepcidin. These results show that there is poor correlation between urine and serum hepcidin levels, there is a diurnal variation of hepcidin levels with concentrations peeking in the early afternoon hours and that the inter-individual differences are significant (Kroot et al., 2009).
Role of hepcidin in various disorders
Several recent clinical studies found connection between hepcidin expressional level and various physiological or pathological conditions where iron metabolism is involved. Roe et al. (2009) demonstrated that inter-individual variability in iron absorption is in a significant part explained by altered levels of circulating plasma hepcidin. Down-regulation of hepcidin occurs in hereditary hemochromatosis (van Bokhoven et al., 2011). It is also believed that hepcidin resistance is responsible for hemochromatosis at least in some of the cases. Moreover, Lin et al. (2013) showed that decreased serum hepcidin was correlated with significant iron deposition in the basal ganglia in cirrhotic patients.
Up-regulation of hepcidin has been also found in many pathological conditions. For example, Sharma et al. (2008) found elevated hepcidin levels in multiple myeloma. Ashby et al. (2009) demonstrated that elevated hepcidin is associated with anemia during chronic kidney disease and that erythropoietin treatment suppressed hepcidin levels to some extent. Ruivard et al. (2009) showed that decreased iron absorption in overweight persons could be attributed to elevated hepcidin levels. Interestingly, malnutrition in anorexia nervosa seems to also increase hepcidin levels (Papillard-Marechal et al., 2012). The influence of metabolic disorders on hepcidin expression might be connected to insulin; it was shown recently that insulin resistance, but not insulin deficiency or hyperglycaemia, is associated with inadequate hepcidin levels in metabolic syndrome (Datz et al., 2013).
Role of hepcidin in the differential diagnosis of anemia and inflammation
A major question is if we need hepcidin measurement in the clinical diagnostic practice at all. The present armament of iron homeostasis determination improved during the past decades and complementing the traditional blood iron level and haematology status now serum transferrin, ferritin, and soluble transferrin receptor (sTfR) analysis is also available. As transferrin delivers iron to the tissues, it behaves in the circulation as a typical export protein. To the contrary, ferritin and sTfR are cellular proteins and, although they can be found in the circulation, this is the result of normal cellular turnover. Typically, in iron-deficiency anemia, serum transferrin level and sTfR increase, while transferrin saturation and ferritin decrease.
The levels of genuine plasma proteins usually decrease in pathological conditions, whereas the concentrations of cellular proteins increase in the blood. It is not surprising that ferritin is also an acute phase protein, which is elevated in the blood in malignancies and infections, including hepatitis. In contrast, transferrin is a negative acute phase protein and its levels decrease upon these conditions, except hepatitis. sTfR measurement was introduced to alleviate inflammation's masking effect on the body's iron status. sTfR is considered less dependent from inflammation; thus, sTfR and especially sTfR/log ferritin ratio is supposed to help assessing iron-status during inflammation; however, measuring sTfR is still very expensive and it is not very specific for iron-deficiency anemia (Koulaouzidis et al., 2009). Briefly, the usefulness of these indicators in real clinical situations is often limited because non-iron homeostasis-related conditions also cause significant alterations.
If and when hepcidin measurements are appropriately standardized, there will be ample room to improve the diagnostic value of clinical laboratories in the differential diagnosis of iron homeostasis. It is important to point out that the above-mentioned biomarkers presently used to asses iron homeostasis are not iron homeostasis regulators but have roles in the iron transport and storage. Using hepcidin as a biomarker, we might have a tool that directly reports about the iron regulation and which is responsive to the changes of iron homeostasis.
Measuring hepcidin might also reveal the underlying causes in therapy-resistant iron deficiency. The most common cause of anemia is iron deficiency, but it does not always respond to iron supplementation therapy. Bregman et al. (2013) described that elevated hepcidin levels are often associated with supplementation refractory anemia. Anemia is frequently in a causal relationship with chronic inflammation, in which case hepcidin elevation may be part of the acute phase response, consequently affecting iron metabolism. Indeed, infused lipopolysaccharides were found to induce up-regulation of hepcidin mRNA expression in an animal inflammation model (Oliveira-Filho et al., 2012). Burte et al. (2013) found that hepcidin in severe malaria is part of the acute phase response but showed little relation with the iron or anemic status. Several conditions related to inflammation has been also shown to impact hepcidin expression levels, consequently influencing iron homeostasis. For example, the severity of hepatitis-C induced liver lesions correlate with hepcidin levels (Tsochatzis et al., 2010). The inflammation-induced elevation of hepcidin is associated with the development of anemia in septic patients (van Eijk et al., 2011). Basseri et al. (2013) showed that hepcidin levels negatively correlates with blood hemoglobin concentration in Crohn's disease. In chronic inflammatory diseases, such as rheumatoid arthritis (RA), the serum concentration of prohepcidin correlates with the disease activity (Swellam et al., 2013), regardless of the actual state of iron stores in the patients.
Serious consideration is given whether hepcidin measurement provides useful data to design iron supplementation therapy in some of the patients with chronic kidney disease (Nakanishi et al., 2012). In chronic kidney diseases complicated with anemia, erythropoietin supplementation resistance is often found to be accompanied with elevated hepcidin levels. Therapeutic strategies aiming to lower hepcidin level may improve gastrointestinal iron absorption by activating ferroportin iron transport (Tsuchiya and Nitta, 2013). However, it appears that hepcidin levels do not respond to erythropoietin stimulating agents but are more influenced by the inflammation status and residual kidney function (van der Weerd et al., 2012), more precisely decreased tubular reabsorption (Peters et al., 2013). Nevertheless, successful attempts have been made to reduce hepcidin levels by dialysis membranes (Kuragano et al., 2013), anti-hepcidin antibodies (Cooke et al., 2013), or by hypoxia inducible factors (Liu et al., 2012).
CNS disorders
Because of the existence of the blood–brain-barrier (BBB), iron has to travel through the brain microvascular endothelial cells (BMCEs) (Mills et al., 2010). The uptake occurs via the endocytosis of the iron-loaded Tf bound TfR complex, then ferroportin and probably DMT1 are also involved in the release of iron to the (brain) interstitium (Gao and Chang, 2014). Oligodendrocytes synthetize and release transferrin to carry iron in the cerebrospinal fluid, while neurons and glia cells are expressing TfR and thus able to take up iron (Crichton et al., 2011). There are probably other routes for iron to enter neuronal cells; for example, iron carried by small molecular complexes could pass through the blood–brain-barrier. Similar to other peripherial tissues, the nervous system also produces heme or iron–sulphur cluster containing proteins, but there are important, unique biosynthetic pathways specific for the CNS that require the presence of iron (neurotransmitter and myelin syntheses) (Stehling et al., 2014). It is not known yet how excess iron can leave the CNS via the cellular iron-exporter ferroportin. It has been assumed that through the interstitium and the cerebrospinal fluid iron may freely enter the blood stream (Crichton et al., 2011).
The CNS has an extremely high iron demand. The cells of the central nervous system have a high demand of iron because of heme and iron–sulphur cluster synthesis (complexes of terminal oxidation system), neurotransmitter synthesis, DNA and protein synthesis at synapses, continuous myelin synthesis (Mills et al., 2010). Lower than normal iron level is especially dangerous during the development of CNS, as iron deficient anemia has been reported to cause cognitive impairment in childhood (Hare et al., 2013). On the other hand, iron accumulation in certain brain areas may often occur in elderly patients (Wang et al., 2010), which may induce or aggravate neurodegenerative diseases. Though the components of iron transport are the same in the CNS as in the peripheral tissues (Tf, TfR1/2, DMT1, ferritin, coeruloplasmin, ferroportin, hepcidin), the regional and intracellular distribution of these proteins varies between brain cells (Zechel et al., 2006). Neurons, glial cells (astrocytes, oligodendrocytes, ependymal cells), and microglia all show subtle differences in the regulation of iron metabolism.
Hepcidin is expressed practically in every region of the brain in both neurons and glial cells (Wang et al., 2010). Microglia has a detectable level of intracellular hepcidin when stimulated (inflammatory signals or iron burden) (Urrutia et al., 2013). In the CNS, it seems that hepcidin fulfils a local regulatory function (McCarthy and Kosman, 2014). The expression level of hepcidin increases with age and by inflammatory stimuli. High level of hepcidin will cause diminished expression of ferroportin and elevated level of DMT1 (Urrutia et al., 2013), consequently intracellular iron accumulation will increase, though lately, these effects are questioned (Du et al., 2014). In vulnerable regions of the brain, which have a higher tendency to accumulate iron, iron deposits might occur (e.g., substantia nigra) (Wang et al., 2008). Neuronal cells are also exceptionally vulnerable to iron overload; in neuronal cells, the mitochondrial ferritin level is high (Gao and Chang, 2014) and mitochondrial terminal oxidation (ATP synthesis) is very intensive. Higher level of mitochondrial iron generates excess of oxygen radicals that might cause or exacerbate neurodegenerative diseases.
The relationship between iron metabolism and neurologic disorders has been known for a long time. Increasing number of evidences suggest primary or secondary pathogenic role for the altered iron metabolism in Parkinson's (Massoura et al., 2011) and Alzheimer's diseases (Tao et al., 2014a). Iron also plays a central role in the pathophysiology of neuroferritinopathy and Friedreich's ataxia (Vaubel and Isaya, 2013). There is a growing number of evidence showing that hepcidin may be involved in the pathogenesis of these disorders, as the level of hepcidin and ferroportin has been shown to be reduced in Alzheimer's disease in some cases (Raha et al., 2013). Nevertheless, definitely more research need to be done to clarify the exact role of hepcidin in the regulation of iron in the nervous system.
Malignant diseases
Multiple studies proved that iron homeostasis is disturbed in a number of various neoplastic diseases. Breast cancer (Lamy et al., 2014), non-Hodgkin's lymphoma (Tisi et al., 2014), hepatocellular carcinoma (Abd Elmonem et al., 2009), multiple myeloma (Mei et al., 2014), prostate cancer (Tanno et al., 2011), ovarian cancer, and upper gastrointestinal tract tumours (Maccio et al., 2015) have been all shown to be associated with increased serum hepcidin level. The intracellular iron excess from colorectal cancer, most likely due to increased systemic hepcidin, are associated with overproduction of IL-6, STAT-3, TfR2, BMP4. Hepcidin acts directly as prooncogene or indirectly through iron. Iron overload generates reactive oxygen species, which causes chronic inflammation and activates signalling pathways, which plays crucial role in colorectal carcinogenesis (Chua et al., 2010).
Conclusions
The primary regulatory function of hepcidin in iron metabolism is now firmly established. Despite some remaining controversies, the effect of hepcidin has been gradually uncovered at the levels of molecular interactions and their physiological and pathological consequences. One of the potential practical benefits of hepcidin level determination is to help the diagnosis of various conditions related to altered iron metabolism (Table 2). Due to unexpected analytical difficulties of hepcidin measurements, it remains yet to be seen whether it can occupy an accepted position in the armament of currently used iron homeostasis laboratory markers.
| Medical condition | Serum hepcidin level | Reference |
|---|---|---|
| Hereditary hemochromatosis type 2B | Decrease | (van Bokhoven et al., 2011) |
| HBV-related liver cirrhosis | Decrease | (Lin et al., 2013) |
| Hepatitis C-related liver cirrhosis | Decrease | (Tsochatzis et al., 2010) |
| Anaemia of chronic renal disease | Increase | (Ashby et al., 2009) |
| Multiple myeloma | Increase | (Sharma et al., 2008) |
| Dysmetabolic iron overload syndrome | Increase | (Ruivard et al., 2009) |
| Non-Hodgkin's lymphoma | Increase | (Tisi et al., 2014) |
Acknowledgement and funding
This work was funded by the Research Fund of the University of Pecs, Faculty of Medicine (ÁOK-KA 2013/19) and by the New Széchenyi Plan (SROP-4.2.2.A-11/1/KONV-2012-0017). “Identification of new biomarkers, especially, regarding the toxicity of free iron deposition in the nervous system, iron toxicity-induced oxidative stress and innate immune reactions with translational investigations.”




