Comparing the effect of equiaxial cyclic mechanical stimulation on GATA4 expression in adipose-derived and bone marrow-derived mesenchymal stem cells
Abstract
Myocardium is prone to mechanical stimuli among which pulsatile blood flow exerts both radial and longitudinal strains on the heart. Recent studies have shown that mechanical stimulation can notably influence regeneration of cardiac muscle cells. GATA4 is a cardiac-specific transcription factor that plays an important role in late embryonic heart development. Our study aimed at investigating the effect of equiaxial cyclic strain on GATA4 expression in adipose-derived (ASCs) and bone marrow-derived (BMSCs) mesenchymal stem cells. For this reason, both ASCs and BMSCs were studied in four distinct groups of chemical, mechanical, mechano-chemical and negative control. According to this categorisation, the cells were exposed to cyclic mechanical loading and/or 5-azacytidine as the chemical factor. The level of GATA4 expression was then quantified using real-time PCR method on the first, fourth and seventh days. The results show that: (1) equiaxial cyclic stimulation of mesenchymal stem cells could promote GATA4 expression from the early days of induction and as it went on, its combination with chemical factor elevated expression; (2) cyclic strain could accelerate GATA4 expression compared to the chemical factor; (3) in this regard, these results indicate a higher capacity of ASCs than BMSCs to express GATA4.
Abbreviations
-
- DMEM
-
- Dulbecco's modified Eagle's medium
-
- MSC
-
- mesenchymal stem cell
-
- BMSC
-
- bone marrow-derived mesenchymal stem cell
-
- ASC
-
- adipose-derived stem cell
Introduction
Mechanotransduction known as a process during which cells change mechanical stimuli into biochemical signals has been shown to play significant roles in cell biology (Wang and Li, 2010; Holle and Engler, 2011). Micromechanical environment can greatly influence cellular events, such as proliferation, differentiation, and gene and protein expression, in both adult and stem cells (Wang and Thampatty, 2008). Different mechanical stimuli including shearing force (Chen et al., 1999), tension (Ingber, 1997), compression (Szafranski et al., 2004) and pressure (Tschumperlin et al., 2004) have been used as in vitro physiological environments of cells to provide the in vivo conditions they experience. Some studies have used strategies to mimic ECM stretching by imposing extension on silicone (Katsumi et al., 2005) or hydrogel (Ahmed et al., 2010) membranes as the cell substrates. Realizing the effectiveness of mechanical stimulation on cell structure and functionality led researchers to study the role of mechanical environment in the fate of stem cells.
The microenvironment and niche that stem cells encounter could control their behavior (Ohlstein et al., 2004). While the role of chemical mediators and macromolecular factors has been paid much attention, a few have focused on controlling stem cell behavior by mechanical stimulation. Some studies in this area have indicated that stem cells are capable of sensing and responding to the alterations in the stiffness of their microenvironment by being differentiated selectively through different pathways and cell lineages; and also it has been shown that mechanical forces could initiate the differentiation processes through different methods and appliances (Lee et al., 2011). In this regard, mechanotransduction could be an effective factor in stem cells' proliferation and differentiation into fibroblasts (Uysal and Mizuno, 2010), chondroblasts (Sandino et al., 2010), myoblasts (Bayati et al., 2011; Haghighipour et al., 2012), osteoblasts (Nagel and Kelly, 2010) and cardiomyocytes (Bhang et al., 2010).
Myocardial infarction is a major cause of death among people in the world (Li et al., 2007). Many studies in regenerative medicine have focused on making use of stem cells in different ways through cardiomyogenesis by different methods. Different approaches towards stem cell differentiation into cardiomyocytes have been reviewed (Heng et al., 2004), including development of a suitable culture medium for the desired differentiation, use of extracellular matrix, co-culture and cell-conditioned media and physical stimuli in the form of electrical pulses, mechanical forces and heat treatment. The idea of making use of mechanical forces in cardiomyogenic differentiation rose when the vital role of mechanotransduction in controlling cardiomyocytes was revealed. Vandenburgh et al. (1995) reported that passive mechanical stretch loading on neonatal cardiomyocytes could cause the upregulation of myosin heavy chain expression and organisation of the cardiomyocytes into parallel rod-shaped cells. However, a similar experiment on adult cardiomyocytes explained that passive loading could not stimulate this kind of cells effectively (Decker et al., 1997). Later, other findings indicated that contractile mechanical stretching could upregulate protein synthesis in adult cardiomyocytes (Wada et al., 1996) and cause secretion of growth factors in neonatal cardiomyocytes (Ruwhof et al., 2000). There are positive influences of electrical stimulation (Serena et al., 2009), laminar shear stress (Huang et al., 2010) and pulsatile perfusion (Morsi et al., 2007) bioreactors on heart muscle formation in vitro. However, most of them have recognised exposure to cyclic mechanical stress as the promising approach (Gwak et al., 2008; Bhang et al., 2010; Huang et al., 2012), and less consideration has been dedicated to the equiaxial form of cyclic strain, which can be obtained by applying the mechanical strain to adherent cells on a circular deformable substrate (Park et al., 2007).
GATA4 is the zinc finger-containing transcription factor, highly expressed in cardiomyocytes through the entire procedure of heart development, which is an essential regulator of cardiomyogenic differentiation (Oka et al., 2007). It is a transcriptional mediator that responds to mechanical force and direct stretching of the ventricles in the isolated heart could activate GATA4 (Hautala et al., 2002). By increasing GATA4 activity, an angiogenic program would be induced in hearts with a myocardial infarction (Heineke et al., 2007). GATA4 expression is upregulated in BMSCs under shear stress (Huang et al., 2010) and uniaxial cyclic stress (Huang et al., 2012).
One of the other important factors is that the source of stem cells plays a significant role in the efficacy of the experiments and treatments (Laake et al., 2006). Orlic et al. (2002) have compared different kinds of stem cells including embryonic and fetal stem cells, adult mesenchymal stem cells (MSCs), and adult hematopoietic stem/progenitor cells with each other and have deduced that MSCs show most promise for heart repair. MSCs are believed to be the multipotential stem cells that could give rise to cardiac myocytes in the heart; their transplantation would not result in neoplasia as may coexist with other stem cell types. Adipose-derived mesenchymal stem cells (ASCs) are a group of MSCs which are believed to have the ability to differentiate into various cell types just as bone marrow-derived mesenchymal stem cells (BMSCs) do; however an advantage of ASCs over BMSCs is claimed to be its being accessible in large quantities with a faster proliferation rate in vitro (Xishan et al., 2012). When it comes to their comparison in their functionality in heart repair, both of these stem cell types lack tolerance against the cardiac environment (Van der Bogt et al., 2009); however Paul et al. (2011) found that ASCs could improve cardiac function after infarction better than BMSCs. They have also proved the superior viability of ASCs under stressed hypoxic conditions and their higher potential in adopting cardiomyocytes phenotype compared with BMSCs.
In this study, the effect of cyclic strain on both ASCs and BMSCs on GATA4 expression has been followed in different days and a comparison made between the results obtained from the experiments on these two kinds of MSCs and also the variations in the level of GATA4 expression in the three different days of induction. For this purpose, MSCs were isolated from rabbit bone marrow and fat tissue; equiaxial cyclic strain was exerted on both ASCs and BMSCs in the presence or absence of chemical factors (biological growth factor). The level of GATA4 expression was examined by Real-time PCR method on the first, fourth and seventh day after the induction.
Materials and methods
Isolation and culture of BMSCs
Adult white male New Zealand rabbits weighing 2.0–2.5 kg were used. Animal experiments were carried out according to the approved principles of the Ethics Committee of Small Animal Teaching Hospital (SATH), University of Tehran, Iran. About 7–9 mL of bone marrow samples were aspirated from the iliac crest of the rabbits under general anesthesia with a bone biopsy needle on 10-mL heparinised (700 U in 1 mL of sample) syringes under sterile circumstances and kept on ice.
Each sample was diluted at 1:1 by DMEM: F12(1:1) (Sigma) supplemented with 30 µL/100 mL penicillin (100 U/mL) (Sigma), streptomycin (100 µg/mL) (Sigma) and L-glutamine (1%) (Gibco). Samples were then homogenised by pipetting the mixture and throwing away the clots in the sample. Each 4 mL of the diluted bone marrow was transferred gently into a 15-mL centrifuge tube (Falcon) containing 2.5 mL percoll (Sigma). Samples were centrifuged at 400 rcf for 30 min. Four layers formed as the result of sample centrifugation; the layer above the percoll was aspirated, recentrifuged at 1,000 rcf for 10 min. The supernatant was thereafter discarded. The pellet formed at the bottom of the tube was washed by HBSS (Sigma) and was re-centrifuged at 500 rcf for 5 min twice. After the last centrifugation and discarding the supernatant, the pellet containing the desired cells was suspended in the pre-mentioned culture medium supplemented with 10% fetal bovine serum (FBS) (Gibco) and incubated at 37°C in air plus 5% CO2. After 48 h, the cells were washed with warm sterile phosphate-buffered saline (PBS) (Sigma) and the medium replaced with a fresh one, which was changed every 3 days. When cells reached 80% confluency, they were detached using trypsin (Sigma) containing 0.1% EDTA (Sigma) and cultured again in DMEM supplemented with 10% FBS.
Isolation and culture of ASCs
Interscapular fat was harvested in a surgical procedure and stored in cold sterile DMEM under sterile circumstances. The adipose tissue was washed with PBS for three times and cut into small pieces. To digest it enzymatically, fat pieces were incubated in 0.1% collagenase type I (Gibco) at 37°C and air plus 5% CO2 with vigorous shaking every 10 min in order to disperse the adipose tissue effectively. After 30 min of incubation, DMEM supplemented with 20% FBS was added to the partially digested tissue to stop collagenase activity. The undigested tissue was removed and the samples centrifuged at 2,000 rpm for 5 min. After further shaking for assisting the process of separating ASCs from adipocytes, it was again centrifuged at 2,000 rpm for 5 min. The supernatant was removed and the pellet suspended in DMEM supplemented with 20% FBS, 1% penicillin/streptomycin and 1% L-glutamine in a conventional flask before incubation. After 24 h, the medium was replaced by one lacking antibiotics. Changing the medium every 3 days and passaging cells were carried out in the same way as mentioned for the BMSCs. After reaching passage 3, cells were characterised using flowcytometry assay, followed by a multipotency assay. Cells were treated by mechanical/chemical stimuli.
Cell characterisation
Flowcytometry assay was used for characterizing stem cells by following the surface antigens CD44 and CD90 as positive markers and CD45 as negative marker. The BMSCs and ASCs from passage 3 were trypsinised, counted and aliquoted in different centrifuge test tubes (500,000 cells per tube) followed by centrifugation at 500 rcf for 5 min. The antibodies were diluted by PBS at 1:100. The cells were incubated at room temperature with the following antibodies: Mouse anti-rabbit CD44 (AbD Serotec), Mouse anti-rabbit CD45 (AbD Serotec), Mouse IgG1 negative control (AbD Serotec) for 30 min and Pierce CD90 Monoclonal Antibody, FITC conjugate (OX-7) (Thermo Fisher Scientific) for 1 h in darkness. After washing the cells with PBS + 10% FBS, the unconjugated antibodies were incubated with the secondary antibody Rabbit anti-mouse IgG: FITC (AbD Serotec) at room temperature in darkness for 30 min. Unstained cells were also examined to set gating. Finally, the results were analysed using FlowJo Cytometry Analysis Software (version 7.6.4).
Multipotency assay
Adipogenic, osteogenic, chondrogenic and cardiomyogenic differentiation were imposed on both BMSCs and ASCs in distinct groups. For adipogenic differentiation, the cells were cultured in a differentiating medium containing the following substances for 15 days: DMEM supplemented with 500 µM IBMX (isobutylmethylxanthine; Sigma), 5 µg/mL insulin (Sigma), 1 µM dexamethasone (Sigma) and 60 µM indomethacin (Sigma). The formed vacuoles that were rich in lipids were stained using oil red O (Sigma).
Osteogenic differentiation involve exposure of the cultured cells to DMEM supplemented with 10 mM β-glycerophosphate (Sigma), 50 µM ascorbate-2 phosphate (Sigma) and 0.1 µM dexamethasone for 3 weeks. Alizarin red s (Sigma) was used for staining the mineralised colonies.
For promoting chondrogenic differentiation, pellets of 2 × 105 cells were cultured in a medium consisting of DMEM supplemented with 50 µM ascorbic acid-2 phosphate (Sigma), 10 ng/mL TGF β1 (transforming growth factor-β1; R&D Systems), 100 nM dexamethasone, 1% ITS-Premix (BD Biosciences) and 1 mM sodium pyruvate (Gibco) for 4 weeks. Chondrogenic differentiation was assessed by fixing the resulting nodules in 10% formalin (Sigma), sectioning and staining them by Alcian blue (Sigma).
In order to perform cardiomyogenic differentiation, cells were cultured in fresh DMEM supplemented with 10% FBS and 10 µM 5-azacytidine (Sigma) for 24 h. Then the medium was completely removed and after washing cells with PBS for three times, they were cultured in pure DMEM. After 5 days, the upregulation of Connexin43 expression as a heart-specific marker was investigated using connexin43 antibody (Abcam) by immunocytochemistry method.
Application of chemical and mechanical stimuli
Samples in three test groups were exposed to mechanical, chemical and mechano-chemical stimuli. Tests were performed for BMSCs and ASCs separately. Undifferentiated BMSCs and ASCs were taken as negative control groups. Circular silicone membranes were coated with collagen type I solution (Sigma) (0.8 mg/mL in 0.1% acetic acid). Cells were cultured in the center of the membranes with DMEM supplemented with 10% FBS. After reaching a confluency of 70–80%, differentiation was induced in different groups as described below.
Differentiation by chemical stimulus
In the chemical and mechanochemical groups, cells were first incubated in the presence of DMEM supplemented with 10% FBS and 10 µM 5-azacytidine as the differentiating factor for 24 h. Cells were washed with PBS twice to remove the differentiating medium completely. The medium was replaced by a pure and fresh DMEM.
Differentiation by mechanical stimulus
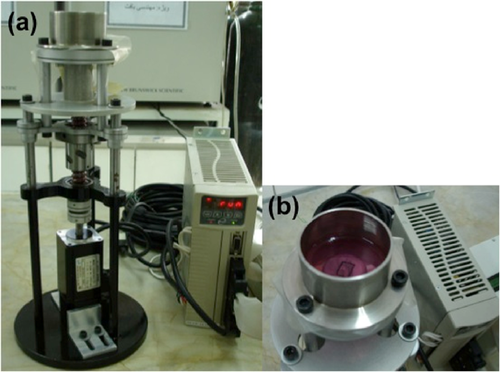
Samples of the mechano-chemical group (immediately after removing the differentiating medium) and the mechanical group were subjected to cyclic equiaxial strain (Park et al., 2007), using a custom-built apparatus manufactured at Pasteur Institute of Iran (Figure 1). The tensile device consisted of mechanical and electrical units. The electrical unit consisted of a custom-made human machine interface (HMI) for enabling the user to adjust mechanical characteristics in each experiment. The mechanical unit contained coupling, ball-screw supports, guides, gripper and medium container. The cell-seeded membrane was fixed at the bottom of the medium container and a shaft beneath the membrane was used to apply cyclic equiaxial strain by the upward and downward movement of the container. Before each experiment, the device was adjusted manually at zero position, which defined the shaft initial position where the unstretched membrane is in contact with the shaft. The parameters such as frequency, time and strain were then set accordingly through HMI unit. By initializing the movement of the shaft, the membranes and thus the cells were exposed to this mechanical force and consequently the cyclic strain. A 10% cyclic planar strain at 1 Hz for 24 h (Huang et al., 2012) was applied on incubated cells. Undifferentiated stem cells (negative control group) and chemically treated cells (chemical group) cultured on collagen coated membranes were placed in the device but not stimulated mechanically. Differentiation assay in each test group was performed within 1, 4 and 7 days.
Real-time PCR assay
Immediately after the given days, total RNA was extracted by the use of Rneasy mini kit (Qiagen) and stored in RNase free water at −80°C. cDNA synthesis used using QuantiTect® Reverse Transcription kit (Qiagen). Primer express software (version 3.0) was used to design primers and probes based on Taqman® Real-time PCR. The sequences of the primers and probes were designed as shown in Table 1. The HPRT marker was used as the housekeeping gene. One step Real-time PCR was carried out using Taqman® Real-time PCR master mix (Applied Biosystems), with Real-time PCR step one (Applied Biosystems). The PCR tests were carried out in triplicate in five groups of negative control, chemical, mechanical, mechano-chemical and rabbit cardiomyocytes as the positive control. To analyse the data, the method of comparative threshold cycle (CT) was used. The relative changes in a special target gene expression were calculated by the equation 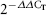 in which ΔCT = CT of target gene − CT of housekeeping gene (normalisation) and ΔΔCT = ΔCT of sample − ΔCT of Calibrator (control group).
in which ΔCT = CT of target gene − CT of housekeeping gene (normalisation) and ΔΔCT = ΔCT of sample − ΔCT of Calibrator (control group).
| Name | Sequence | |
|---|---|---|
| GATA4 | Forward | 5′-CCGGTCCTCCACAGCCT-3′ |
| Reverse | 5′-CCGCAGTTGACACATTCTCTG-3′ | |
| Probe | 5′-CGACACCCCAATCTTGACATGTTTGATG-3′ | |
| HPRT | Forward | 5′-TCGAGGACTTGGAAAGGGTG-3′ |
| Reverse | 5′-CCTCCCATCTCCTTCATCACAT-3′ | |
| Probe | 5′-TGGACTCATTATGGACAGGACTGAAAGGC -3′ | |
The data were presented as mean ± SD from three independent experiments performed in triplicate. The results have been compared with those of the control group, with the mean values determined with the t-test. Significance was set at P < 0.05.
Results
Characterisation and multipotency of BMSCs and ASCs
Flowcytometry showed high purity of stem cells in the 3rd passage cell culture (Table 2).
| CD marker | Expression | |
|---|---|---|
| BMSCs | ASCs | |
| CD44 | 97.60% | 93.52% |
| CD90 | 99.96% | 98.11% |
| CD45 | 0.19% | 3.87% |
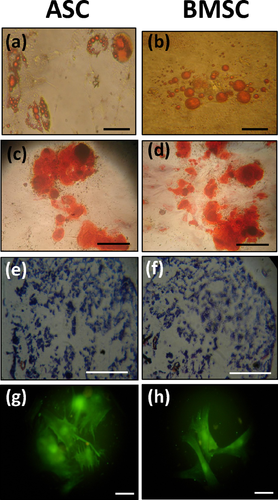
The multipotency test showed formation of lipid droplets on the 7th and 12th days of culture for ASCs and BMSCs, respectively. After 15 days, oil red O stained lipid vacuoles (Figures 2a and 2b). After 3 weeks, the cells that were exposed to osteogenic medium were stained by alizarin red s to characterise the calcified colonies (Figures 2c and 2d). After 4 weeks, the sulfated glycosaminoglycan in BMSCs and ASCs was stained by alcian blue (Figures 2e and 2f). Upregulation of connexin 43 expression was also examined by immunocytochemistry test on day 5 (Figures 2g and 2h). These multilineage differentiation experiments confirmed the multipotency of the isolated cells.
GATA4 mRNA expression
The results from the Real-time PCR tests are given in Figures 3 and 4. The charts (Figure 3) indicate the amount of GATA4 gene expression relative to HPRT (house-keeping gene) compared to the negative control group in three groups of chemical, mechanical and mechano-chemical (the level of GATA4 expression in the mRNA extracted from the rabbit cardiomyocytes relative to HPRT and compared to its expression in BMSCs and ASCs was 96.3 and 140.2, respectively, and has not been included in the result graphs). Figure 4 is a reconfigured form of Figure 3 with the aim of comparing the gene expression in different days.
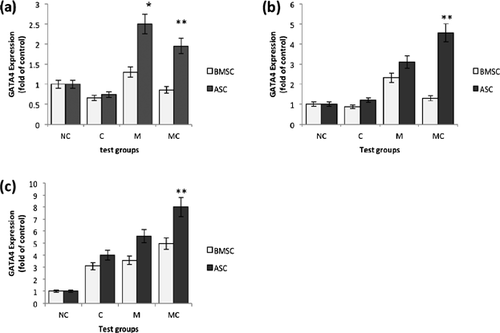
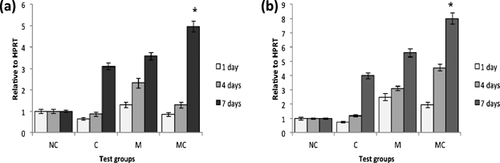
Mechanical stimulation increased the value of GATA4 expression significantly in the cells subjected to mechanical loading compared to the negative control cells (P < 0.05). This upregulation was further enhanced when accompanied by chemical factor in the mechano-chemical group on the 4th and 7th days of the tests (Figures 3b and 3c; P < 0.05). However, the graphs show that the expression in the chemical group was downregulated on the first day (P < 0.05). It seems that this effect has also influenced the results of gene expression in the mechano-chemical group cells on this day; as a result, decreased GATA4 expression is seen compared to the solely-mechanically stimulated cells. ASCs express GATA4 to a higher level than BMSCs in each test group (P < 0.05). In general, the positive effect of mechanical stimulation on GATA4 expression was more prominent in ASCs than BMSCs (Figure 3). Moreover, a general trend of increase in the level of gene expression with time in the entire test groups was noted (P < 0.05; Figure 4).
Discussion
Once the formation and function of heart begins, it encounters both mechanical deformations and fluid flow (Patwari and Lee, 2008). The heart beat initiates during the last steps of the process of heart tube formation, which is the reason for the subsequent steps being claimed as affecting myocardial function (Bartman and Hove, 2005). Although dramatic changes occur during heart genesis, the main function of heart is preserved. This results in a biophysical stimulation and several types of forces exerted frequently on this organ including shear stress caused by the friction between blood and wall surface of the heart, pressure caused by the blood as the result of heartbeats, and cyclic stretch created by pulsatile blood flow.
The positive effects of some kinds of mechanical stimuli, such as shear stress (Huang et al., 2010) and uniaxial cyclic stress (Schmelter et al., 2006; Huang et al., 2012), the upregulation of GATA4 expression as an essential transcription factor and mechanical load-responsive transcriptional mediator in cardiogenesis (Heineke et al., 2007), have been seen in previous studies. However, less attention has been dedicated to the consequences of cyclic equiaxial stretch. The effects that mechanical stimulation may have on cardiogenic differentiation could also vary with different stem cell types. This deduction seems to be based on the different results that have been reported in some in vivo experiments on various untreated stem cell types used in heart repair (as reviewed in Orlic et al., 2002).
Our experiments on cyclic equiaxial mechanical stimulation on GATA4 expression in bone marrow-derived and ASCs show that in their entire duration, this stress upregulates GATA4 expression in both BMSCs and ASCs compared to untreated cells (P < 0.05). This is consistent with previous data on effects of uniaxial tension. According to Huang et al. (2012), a significant increase in GATA4 expression is detected when MSCs are subjected to uniaxial cyclic strain for 7 days with the same conditions as in our study. Our data show that the levels of GATA4 expression induced by mechanical stimulation in the cells surpass even those initiated by chemical stimulation. However, another study concluded that cyclic equibiaxial stimulation alone cannot affect mRNA levels of GATA4 (Guo et al., 2011). This may be due to the lower tensile strain (8%) or higher period of mechanical stimulation (10 days of mechanical loading) that was used. In this regard, it has also been stated that the level of GATA4 expression reached its highest value when subjected to 10% uniaxial strain (Huang et al., 2012).
Within the chemical group, a downregulation in the level of GATA4 expression compared to the negative control was seen in both BMSCs and ASCs on the first day of induction. The decrease in the mRNA level of GATA4 on the first day of induction could be interpreted as a sudden toxic effect of 5-azacytidine (Matoušová et al., 2011). The reduction in GATA4 expression seems to have affected the amount of GATA4 expression in the mechano-chemical group as well. After removal of the medium containing 5-azacytidine on the first day, the expression increased on the fourth day and rose even more on the seventh day in both types of stem cell. Thus on the first day downregulation of GATA4 expression, which has led to its decrease in the mechano-chemical group, was compensated by upregulation of GATA4 expression in the chemical group. This happened on the fourth day for ASCs and on the seventh day for BMSCs. However, upregulation of GATA4 expression was first markedly so on the seventh day (or perhaps in the interval of 4–7 days) of chemical stimulation in the both types of the stem cells. GATA4 expression can be seen in similar experiments on the sixth (Choi et al., 2004) and seventh days (Kawada et al., 2004; Yang et al., 2012). In general, >5 days of induction, mechanical stimulation combined with 5-azacytidine could accelerate cardiomyogenic differentiation in the mechano-chemical group. Guo et al. (2011) have pointed to the higher effect of mechanical loading accompanied by chemical factors on cardiac gene expression than that of the each of the stimulators alone.
Upregulation of GATA4 expression rose by increase of duration of treatment in both BMSCs and ASCs for all test groups in our experiments. GATA4 as an early cardiac transcription factor, which is one of the first expressed genes during embryogenesis (Liang et al., 2001) and an important gene in late embryonic heart development (Zeisberg et al., 2005; Oka et al., 2006), maintains its expression until the final differentiation of cardiomyocytes (Fijnvandraat et al., 2003). GATA4 expression is initiated at the very beginning of cardiomyogenic differentiation and begins to increase until reaching a plateau on day 7 of the differentiation (Huang et al., 2010). Such an increase in GATA4 expression depicted in this study is consistent with current results.
The efficacy of mesenchymal stem cells (BMSCs) with different origins in vascular regeneration has been reviewed (Huang, 2008), and BMSC has been defined as a promising cell type for cardiomyogenic differentiation. Uniaxial cyclic strain combined with 5-azacytidine upregulates GATA4 expression in BMSCs (Huang et al., 2010) and ASCs (Guo et al., 2011). GATA4 in our work has a higher expression in ASCs compared than BMSCs in all test groups. This may be interpreted as the potency of ASCs to accelerate cardiomyogenic differentiation under special chemical and/or mechanical stimulation. ASCs may have a stronger potential in proliferation and multi-lineage differentiation in vitro compared to BMSCs (Martinez and Kofidis, 2011). In vivo ASCs have some advantages over BMSCs in higher resistance and proliferation in stressed hypoxic conditions in heart, and also significant improvement in heart function and reduced infarction (Paul et al., 2011).
In conclusion, our novel approach has been to compare the effect of cyclic equiaxial strain on GATA4 expression with or without chemical differentiation factors in ASCS and BMSCs. The results indicate that this strain can potentiate GATA4 expression within the two major sources of MSCs. Mechanical stimulation may accelerate GATA4 expression compared to the chemical factor and its combination with chemical stimulation could enhance expression. In this regard, ASCs have a better efficacy in cardiomyogenic differentiation than BMSCs. Although the expression of GATA4 as a cardiac-specific transcription factor, and even its upregulation when exposed to mechanical stress, does not guarantee the functionality of the treated cells, and might assist in exploring efficient methods in achievement of functional cells in cardiovascular engineering.
Acknowledgement and funding
This work has been financially supported by Iran Stem Cell Technology Organisation and carried out in NCBI, Pasteur Institute of Iran.