Therapeutic effects of hepatocyte growth factor-overexpressing human umbilical cord blood-derived mesenchymal stem cells on liver fibrosis in rats
Abstract
Fibrosis is a common end stage for a variety of liver diseases, including most chronic liver diseases, and results from an imbalance between collagen deposition and degradation. Mesenchymal stem cells (MSCs) have the ability to migrate into fibrotic livers and differentiate into hepatocytes. Hepatocyte growth factor (HGF) has potent anti-apoptotic and mitogenic effects on hepatocytes during liver injury and plays an essential role in the development and regeneration of the liver. In this study, human HGF-overexpressing human umbilical cord blood-derived MSCs (hHGF-HUCB-MSCs) were prepared using the pMEX Expression System, and the upregulation of hHGF expression was confirmed by RT-PCR and ELISA. HGF expressed by hHGF-HUCB-MSCs exerted a stimulatory effect on hepatocyte proliferation in vitro. hHGF-HUCB-MSCs were transplanted to investigate the therapeutic effects of these cells on carbon tetrachloride (CCL4)-induced liver fibrosis in a rat model. After 4 weeks of cell treatment once per week with 2 × 106 cells, biochemical analysis of the serum and histopathological analysis of the liver tissue were performed. The results of the biochemical analysis of the serum show that the hHGF-HUCB-MSC-treated group had higher levels of alanine aminotransferase, aspartate aminotransferase and alkaline phosphatase, indicating the improvement of liver function. Histopathology showed that the hHGF-HUCB-MSC-treated group had reduction in the density of collagen fibres. Thus hHGF-HUCB-MSCs can enhance liver regeneration and could be useful for the treatment of patients with liver fibrosis or cirrhosis.
Abbreviations
-
- HGF
-
- hepatic growth factor
-
- HUCB
-
- human umbilical cord blood
-
- MSC
-
- mesenchymal stem cell
Introduction
Liver cirrhosis is a major cause of morbidity and mortality worldwide for which there is no effective therapy, although if treated properly at the fibrosis stage, cirrhosis can be prevented (Pinzani and Rombouts, 2004). Liver fibrosis, a common pathological process in individuals with chronic hepatic disease, involves multiple cellular and molecular events that ultimately result in the accumulation of collagen and extracellular matrix (ECM) (Hong et al., 2009). This wound-healing process is initiated by hepatocyte damage, the activation of hepatic stellate cells (HSCs) and the subsequent release of cytokines, such as transforming growth factor β1 (TGF-β) (Blomhoff and Wake, 1991; Friedman, 1993). The overproduction of TGF-β1 is a major cause of tissue fibrosis in various organs (Blomhoff and Wake, 1991; Friedman, 1993). TGF-β1 induces the phenotypic transition of HSCs into proliferating myofibroblast-like cells, and this transition enhances the production of ECM components and attenuates the degradation of ECM proteins (Nakamura et al., 1985; Border and Noble, 1994; Sanderson et al., 1995).
Mesenchymal stem cells (MSCs) are a diverse population of cells that can be isolated from multiple tissues, including bone marrow (BM), fat and others. The ability of MSCs to differentiate into multiple cell types and the relative ease of expansion makes them attractive treatment candidates under a variety of conditions. Human umbilical cord blood (HUCB) may be preferable to either BM or neural tissue as a source of cells for transplantation because HUCB contains greater numbers of MSCs than BM or adult peripheral blood (Nonome et al., 2005; Yoshida et al., 2007). HUCB is also easy to obtain from live donors, is available for collection after delivery, and remains viable after long-term cryopreservation (Jung et al., 2009).
Previously, novel strategies for the treatment of liver cirrhosis that can boost the therapeutic potential of MSCs through the co-administration of certain growth factors or cytokines have been proposed (Yang et al., 2006). The multi-lineage potential of MSCs makes these cells a promising source for stem cell therapy and gene therapy applications, and the long lifespan and homing ability of MSCs are attractive assets in the context of gene therapy (Jorgensen et al., 2003). The therapeutic potential of HUCB-MSCs for treating liver cirrhosis has also been noted (Alvarez-Mercado et al., 2009; Jung et al., 2009; Bassiouny et al., 2011; Kim et al., 2011; Zhang et al., 2012).
The potential therapeutic effects of hepatocyte growth factor (HGF) on liver injury are known (Okano et al., 1997; Tahara et al., 1999; Xue et al., 2002). HGF, first identified as a potent mitogen for mature hepatocytes (Nakamura et al., 1986, 1989; Gohda et al., 1988), is considered a major factor active in the development and regeneration of the liver (Michalopoulos and DeFrances, 1997). HGF has multiple biological properties, including mitogenic, morphogenic (Matsumoto and Nakamura, 1992; Boros and Miller, 1995; Ricci et al., 1999), and anti-apoptotic activities in a variety of cell types (Kosai et al., 1999; Liu, 1999). HGF has great potential as an anti-inflammatory, anti-fibrosis, anti-apoptotic (Kellermann et al., 2009) and angiogenic agent (Ishizawa et al., 2004). These cellular effects of HGF may increase the therapeutic effect of MSCs for the treatment of liver failure. HGF gene therapy can stimulate liver cell proliferation and promote the recovery of liver function after the resection of cirrhotic areas (Xue et al., 2003). HGF can also promote the remodeling of fibrotic liver in a murine model and accelerate the recruitment of BM-derived cells to the liver (Asano et al., 2007).
This study set out to determine whether human HGF (hHGF)-overexpressing HUCB-MSCs can induce functional and morphological improvements in a rat liver fibrosis model.
Materials and methods
Preparation of cells and cultures
HUCB-MSCs acquired from the Gachon University of Medicine and Science (Incheon, Republic of Korea) were cultured in alpha-Minimum Essential Medium (Hyclone, Logan, UT) containing 15% FBS (Invitrogen, Carlsbad, CA), penicillin (100 U/ml), and streptomycin (100 µg/mL). The cultures were incubated at 37°C in a humidified air atmosphere with 5% CO2. HepG2 adherent epithelial-like cells derived from a human hepatoblastoma obtained from the Korean Cell Line Bank (KCLB, Chongnogu, Seoul, Republic of Korea) were used at passage 94. The cells were maintained in MEM (Hyclone, Logan, UT) supplemented with 10% FBS as above.
Construction of a non-viral DNA expression vector and generation of hHGF-overexpressing hHGF-MSCs
The pMEX-HGF plasmid (Dualsystems Biotech, Zurich, Switzerland) was constructed by inserting the full-length cDNA of human HGF (2.2 kb) into the SalI sites, and was dissolved in endotoxin-free TE buffer. HUCB-MSCs were plated in a six-well plate at 2 × 105 cells/well 24 h prior to gene transfection. The pMEX-hHGF plasmid was transfected into MSCs using Lipofectamine™ 2000 (Invitrogen). Lipofectamine (20 µL) and plasmid DNA (15 µg) were diluted in 1 mL of serum-free medium, followed by equilibration at room temperature for 5 min after mixing. Lipofectamine-DNA complex was added to MSCs, and the cells were incubated for 6 h. The cells were washed with PBS and incubated in MEM containing 10% FBS. After 24 h, the medium was removed, and the cells were cultured with G418 (neomycin, 1,000 µg/mL; Gibco BRL, Grand Island, NY) for 3 days. After selecting transfected hHGF-MSCs, these cells were incubated for 48 h and the supernatant collected as the HGF conditioned, which was centrifuged at 3,000 rpm for 20 min to remove the debris and stored at −80°C until use. The human HGF concentrations of these supernatants were determined by ELISA using an anti-hHGF monoclonal antibody (RayBiotech, Inc., Madison, WI).
Hepatocyte proliferation assay
HepG2 cells were cultured at 5 × 104 cells per well in a six-well plate. After reaching confluence, cultures were divided into three groups, and two of them had the medium changed to 1:1 mixture of MSCs or hHGF-MSCs supernatant and MEM containing 20% FBS. The other group was pre-treated with 20 ng/mL of recombinant human HGF (Sigma–Aldrich, St. Louis, MO). Cell proliferation was quantified by counting trypan blue negative (viable) cells using a haemocytometer.
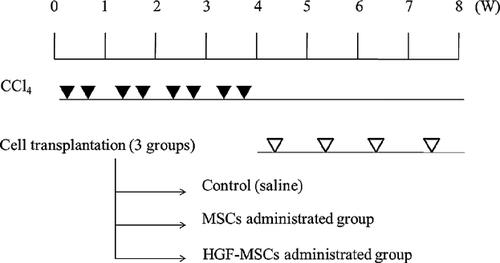
In vivo experimental design for the rat liver fibrosis model
Twenty-four 4-week-old male Wistar rats (Orient Bio, Sungnam, Republic of Korea) were allowed to acclimate for 1 week, being maintained under standard environmental conditions (room temperature with a 12 h light-dark cycle) with free access to water and rodent chow. The handling of the animals was performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Institute of Laboratory Animal Resources, Seoul National University (Approval #: SNU-090514-4).
The rats were divided into two groups. Group I (n = 5) served as the negative control (NC). Group II (n = 20) was the carbon tetrachloride (CCl4)-induced liver cirrhosis group. CCl4 (Sigma–Aldrich) was injected ip at 0.5 mL/kg diluted in corn oil (1:1) twice per week for 4 weeks (Terai et al., 2003). The rats with induced liver cirrhosis were divided into two groups according to the material received by transplantation: (1) the control group (PBS), (2) the UCB-MSC-treated group and (3) the HGF-overexpressing UCB-MSC-treated group. The UCB-MSCs and HGF-overexpressing UCB-MSCs, suspended in PBS (100 µL), were labelled with CM-DiI (Invitrogen) before injection, and 2 × 106 cells were injected via the caudal vein once per week for 4 weeks. A schematic detailing the in vivo experiment is presented in Figure 1.
Histopathology staining
The liver was excised and immersed in hexane chilled with dry ice. Cryostat sections of 5 µm were cut and mounted on glass slides. The liver specimens were fixed in 10% formaldehyde, embedded, sectioned and stained with haematoxylin and eosin (H&E) for routine histology. MT staining was used to measure the extent of fibrosis. The degree of hepatic fibrosis was determined using the Knodell fibrosis score, and histopathologic fibrosis was scored as previously reported (0, no fibrosis; I, perivenular and/or pericellular fibrosis; II, septal fibrosis; III, incomplete cirrhosis; and IV, complete cirrhosis) (Jung et al., 2009).
Biochemical parameters
Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) were determined in an automated biochemical analyser (Selectra 2, Merck, Darmstadt, Germany) 4 weeks after the initiation of the cell-based therapies. Blood samples were collected from the abdominal vein using a 25-gauge needle, followed by excision of the liver.
Detection of hHGF mRNA expression
hHGF-overexpressing UCB-MSCs were given 4 times at weekly intervals, and the rats were killed 4 weeks after the first injection. To determine the level of hHGF mRNA expressed by hHGF-overexpressing UCB-MSCs after transplantation, total RNA was extracted from the fibrotic livers of rats using Trizol Reagent (Invitrogen) and cDNA was synthesized using the Superscript first-strand synthesis system (Invitrogen). The cDNA was used as a template for PCR amplification using gene-specific primers for human HGF and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primers for HGF consisted of a forward primer (5′-CCAGAGGTACGCTACGAAGTCT-3′) in the human HGF gene sequence (GenBank accession no. X16323), and the primers for human GAPDH consisted of a forward primer (5′- AAGTGGATATTGTTGCCATC-3′) and a reverse primer (5′- ACTGTGGTCATGAGTCCTTC-3′) in the human GAPDH gene sequence (GenBank accession no. NM_002046). These were used to amplify 327 and 445 bp fragments to analyse the human HGF and GAPDH transcripts, respectively. PCR amplification of the cDNA was performed in an automated thermal cycler (TECHNE, Teddington, UK) in a final volume of 25 µL containing cDNA samples. After PCR, the amplified products were electrophoresed in 1.5% agarose gel and visualized by ethidium bromide staining under UV light illumination. All reactions were performed in triplicate.
Statistical analysis
Statistical differences were analysed for significance by applying a two-tailed Student's t-test. P < 0.05 was considered statistically significant. Statistical analysis used a commercially available statistical software package (SPSS (Statistical Package for the Social Sciences) 13.0 for Windows; SPSS Inc., Chicago, IL).
Results
Generation of human HGF-overexpressing HUCB-MSCs and the stimulatory effect of hHGF on human hepatocyte proliferation
To assess the bioactivity of hHGF-HUCB-MSCs engineered to overexpress HGF following pMEX expression vector-mediated transduction, human HGF gene expression was measured by RT-PCR and ELISA (Figures 2A and 2B). Its mRNA was significantly upregulated in hHGF-HUCB-MSCs incubated for 48 h compared control MSCs (Figure 2A). A substantial amount of human HGF could be detected by ELISA in the culture supernatant of hHGF-HUCB-MSCs incubated for 48 h (Figure 2B).
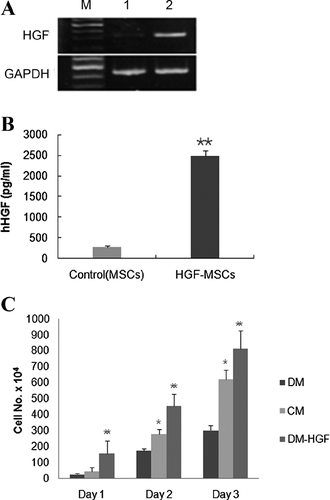
To investigate the effect of HGF produced by hHGF-HUCB-MSCs on hepatocyte proliferation, HepG2 cells were cultured as follows: (1) conditioned media (CM)-1, a 1:1 mixture of the supernatant of HUCB-MSC culture medium from cells incubated for 48 h and minimum essential medium (MEM) containing 20% fetal bovine serum (FBS); (2) CM-2, a 1:1 mixture of the supernatant of hHGF-HUCB-MSC culture medium from cells incubated for 48 h and MEM containing 20% FBS; and (3) CM-3, CM-1 pretreated with 20 ng/mL of recombinant human HGF DM. The human HGF concentration of the medium in each group measured by hHGF ELISA were 0.072, 1.22 and 20 for CM-1, CM-2 and CM-3, respectively. After the HepG2 cells had been cultured for 24, 48 or 72 h under each condition, proliferation was determined by counting the number of viable cells (Figure 2C). Proliferation was markedly promoted by the addition of hHGF in a dose-dependent manner.
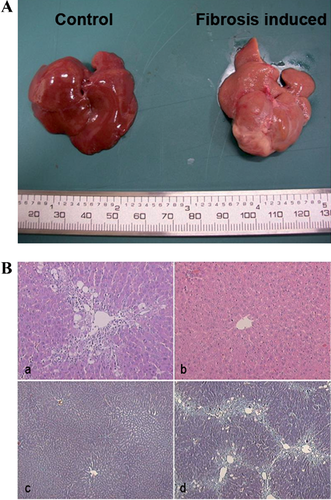
Verification of CCl4-induced liver fibrosis
In the livers of rats that received CCl4, central-central bridging fibrosis and extensive deposition of collagen fibres between the central veins were found. Haematoxylin–eosin (H&E) staining and Masson's trichrome (MT) staining of paraffin sections indicated moderate macrovesicular and microvesicular steatosis along the fibrous septa (Figures 3A and 3B). These observations are consistent with the histological characteristics of liver fibrosis, and the fibrosis scored as grade 3 based on Knodell's scoring system (Table 1).
| Stage score | Description |
|---|---|
| 1 | No fibrosis |
| 2 | Fibrous pericentral expansion |
| 3 | Bridging fibrosis |
| 4 | Fibrosis septa and structural disturbance of hepatic lobule |
| 5 | Cirrhosis |
Assessment of liver function after the transplantation of HGF-UCB-MSCs
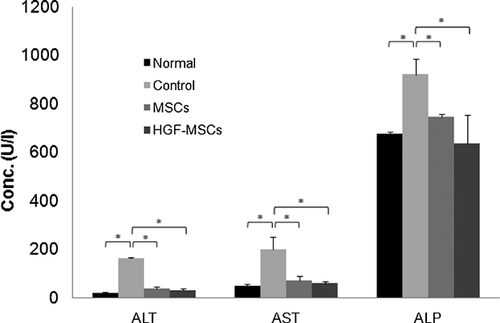
To determine whether HGF-HUCB-MSC transplantation can improve liver function, rats with liver cirrhosis were iv injected with 2 × 106 HGF-HUCB-MSCs once per week for 4 weeks, and the activities of serum alanine aminotransferase (ALT, reference range; 91–97 U/L), aspartate aminotransferase (AST, reference range; 47–56 U/L) and alkaline phosphatase (ALP, reference range; 387–687 U/L) were measured with an automatic biochemistry analyser. ALT, AST and ALP increased in the control group treated with CCl4 relative to the normal group. However, the levels were significantly improved in the MSC and HGF-MSC groups 4 weeks after initiation of cell therapy (Figure 4).
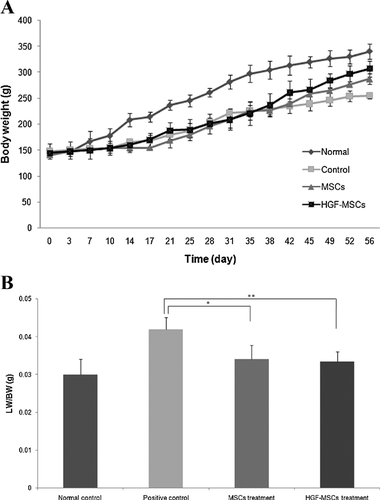
Recovery of body weight and liver weight in the CCl4-induced fibrosis rat model after HGF-HUCB-MSC transplantation
Body weight (BW) was measured over the 56 days following the initiation of the cell-based therapy. After the induction of liver cirrhosis (28 days), the rats were divided into three groups according to the material that they would receive in the transplantation: (1) positive control (PBS), (2) the HUCB-MSC group and (3) the HGF-HUCB-MSC group. The BWs of the rats treated with HGF-MSCs increased to almost the control (normal) value (Figure 5A). The liver weight (LW) per BW was measured 4 weeks after the initiation of the cell transplantation. Only the positive control group had an increased LW. There were no significant differences between the healthy normal group and either the HUCB-MSC-treated group or the HGF-HUCB-MSC-treated group (Figure 5B).
HGF-HUCB-MSCs attenuated CCl4-induced liver fibrosis
H&E staining and MT staining showed the morphological changes and level of hepatic damage in the livers of rats with liver cirrhosis after HGF-HUCB-MSC transplantation. The livers of the rats in the normal group were smooth, lustrous and reddish on the surface, whereas the surfaces of the livers from the rats injected with CCl4 for 4 weeks were coarse and relatively bloodless, with numerous small nodules. In the rats in the MSC treatment group, the liver surfaces were slightly coarse, but were redder and more lustrous than the liver surfaces of the PBS-injected group. The surfaces of liver of the HGF-MSC-treated groups were smoother, redder and more lustrous than those of the MSC-treated group (Figure 6A). To assess the changes in CCL4-induced liver fibrosis after the rats had been treated with MSCs or HGF-MSCs, MT staining was used to identify collagen. Histologically the collagen fibres in the HGF-MSC- and MSC-treated livers were thinner than in the PBS-treated pathologic control group. However, there were fewer collagen fibres in the HGF-MSC-treated group. Both H&E and MT staining indicated that the infusion of HGF-MSCs into rats with CCl4-induced fibrosis improved the histological structure of the liver compared with the control treatment.
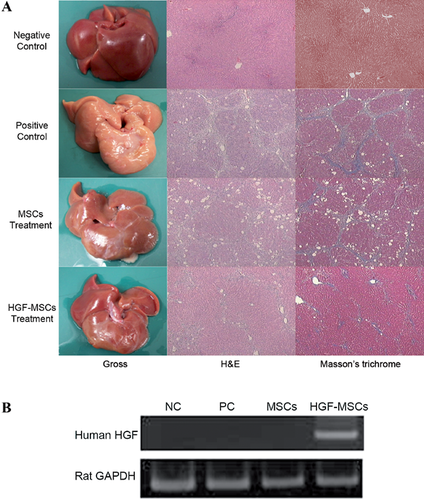
To determine whether the administered HGF-MSCs localize to the liver tissue, the livers from each group were resected, and the hHGF mRNA levels were analysed by reverse transcription polymerase chain reaction (RT-PCR) using a human-specific HGF primer. An abundant expression of hHGF was seen only in the HGF-MSC-treated group (Figure 6B).
Discussion
The effectiveness of hHGF-expressing MSCs in suppressing liver fibrosis and stimulating liver regeneration in vitro and in vivo shows that hHGF-HUCB-MSCs have a powerful therapeutic effect on fibrosis in rats with CCl4-induced cirrhosis. The major findings are as follows: (1) treatment with HUCB-MSCs expressing hHGF improved the liver morphology and reversed liver damage and decreased toxicity, seen as reduction of ALT, AST and ALP back to normal levels; (2) treatment with untransfected HUCB-MSCs delayed the progression of hepatic damage in rats with liver fibrosis; and (3) hHGF-HUCB-MSCs migrated into the injured liver and expressed hHGF.
hHGF-HUCB-MSCs were generated using the pMEX expression system because a non-viral vector system avoids some of the disadvantages of viral vector systems, including immunogenicity, virus-mediated insertional mutagenesis, greater expense and difficulty in manufacturing. Both viral and non-viral systems are available for in vivo gene transfer. Among the viral vector systems, systems based on retroviral vectors have been used for gene delivery into rat livers after partial hepatectomy, with 1–5% of hepatocytes being transfected in this model (Ferry et al., 1991). Adenoviral vectors can induce efficient gene expression in hepatocytes in vivo; however, these vectors can lead to transient gene expression and give some side effects, for example cytotoxicity and immune responses (Li et al., 1993; Yang et al., 1994; Marshall, 1999; Powell et al., 1999; Rabinowitz et al., 1999). Regarding non-viral vector systems, the haemagglutinating virus of Japan (HVJ) liposome method, which is a non-viral vector system, has no side effects (Kaneda et al., 1989a, 1989b; Kato et al., 1991; Matsuno et al., 2003). However, expression of the introduced gene is transient in this system. The highest transfection efficiencies are obtained with viral vectors (Holladay et al., 2010). However, cell-mediated gene transfer is unlike other methods of gene transfer (Holladay et al., 2010), and the transfection efficiency is not really an issue because the cells that do take up the gene are selectively cultured, while those that do not are eliminated. Therefore, a high in vitro transfection efficiency is desirable, but not essential (Holladay et al., 2010). We used the pMEX expression vector, which facilitates high-level, stable target protein expression in mammalian cells. After the transfection of HUCB-MSCs with the pMEX expression vector containing the hHGF gene, the hHGF gene was delivered into HUCB-MSCs and induced markedly high levels of hHGF expression in vitro (Figures 2A and 2B). The in vitro data from the hepatocyte proliferation assay also indicate that the HGF derived from hHGF-HUCB-MSCs stimulates the proliferation of hepatocytes (Figure 2C). Thus hHGF-expressing HUCB-MSCs had been successfully generated.
The effect of HGF in a cirrhotic liver resection model is favourable (Ozawa et al., 2006); HGF has potent cytoprotective effects on hepatocytes and has been used in many experimental and clinical applications (Fujiwara et al., 1993; Ikegami et al., 1999; Tashiro et al., 2003). Among the biological activities of HGF, anti-fibrogenic effects are widely known (Yasuda et al., 1996; Matsuda et al., 1997; Ueki et al., 1999). Unfortunately, exogenous HGF protein is extremely unstable when circulating in the blood (half-life <15 min; Kawaida et al., 1994). Therefore, it is almost impossible to sustain a constant high level of exogenous HGF in the circulation, even when using repeated injections of HGF protein at short intervals. To address this major difficulty with utilizing HGF, vehicles have been developed to transfer therapeutic genes such as HGF that would permit long-term transgene expression (Hu et al., 2010; Motaln et al., 2010).
MSCs are an attractive candidate for liver regeneration/repair, and the BM is a predominant source of MSCs. Evidence from in vitro and in vivo studies has indicated that MSCs have the ability to enhance fibrous matrix degradation and to produce secreted factors that stimulate the regeneration of endogenous parenchymal cells, suggesting that MSCs may be ideally suited for the treatment of liver diseases involving fibrosis (Haynesworth et al., 1996; Fang et al., 2004; Zhao et al., 2004; Zhao et al., 2005; Caplan and Dennis, 2006; Parekkadan et al., 2007; Kuo et al., 2008). MSCs can also differentiate into hepatocyte-like cells and are suitable for transfection with exogenous genes (Hong et al., 2005; Sato et al., 2005; Lange et al., 2006; Ong et al., 2006). Circulating stem cells derived from the BM migrate to the injured liver and contribute to liver regeneration (Gehling et al., 2005).
HUCB contains haematopoietic stem cells and MSCs, both of which can be used as alternatives to BM for cell transplantation and therapy. HUCB-MSCs have many advantages over BM in the procurement and transplantation of cells, such as high abundance, lack of donor attrition, low risk of viral transmission and a less pronounced immune response (Hong et al., 2005), which can lead to better engraftment in non-immunodeficient recipients (Surbek et al., 2008). Regarding liver regeneration, HUCB-MSCs can differentiate into hepatocytes in vitro (Kang et al., 2005; Lee et al., 2004a, 2004b) and in vivo (Newsome et al., 2003); and transplantation of HUCB-MSCs can also suppress fibrotic changes in rat livers with established fibrosis or cirrhosis (Alvarez-Mercado et al., 2009; Jung et al., 2009; Bassiouny et al., 2011; Kim et al., 2011; Zhang et al., 2012).
Based on these findings, we tried to deliver HGF using HUCB-MSCs as carriers to provide targeted and sustainable delivery. RT-PCR analysis of a human-specific HGF primer indicates that the resident hHGF-HUCB-MSCs produce human HGF protein in the fibrotic livers (Figure 6B), suggesting that xenotransplantation of HUCB-MSCs has been successful. MSC xenotransplantation can be beneficial in the repair of tissue injury, and MSCs are poorly recognized by HLA-incompatible hosts due to their limited immunogenicity (Le Blanc et al., 2003), allowing these cells to be used under xenogenous conditions. Human adipose-derived stem cells from young donors administered xenogeneically were not rejected for up to 6 months after transplantation in non-immunocompromised mice (Rodriguez et al., 2005). The therapeutic benefit of HUCB-MSCs was seen in an immunocompetent rat model without graft response (Liao et al., 2009). What both of these studies have in common is that MSC transplanted xenogeneically showed no systemic side effects throughout the course of the experiments. In our study, both non-engineered HUCB and hHGF-HUCB-MSCs were repeatedly injected into immune-competent rats without apparently any side-effects during the course of the experiments. This in vivo result supports the hypothesis that MSC have little to low immunogenicity, making it possible to administer allogeneic MSC without human leukocyte antigen matching for cell therapy. These preceding reports support our data from the in vivo experiments with hHGF-HUCB-MSC transplantation under xenogenous conditions. Transplantation of hHGF-HUCB-MSCs or HUCB-MSCs alone reduces the size of the fibrotic lesions at 4 weeks after injection, similar to the result of Jung et al. (2009). In their study, HUCB-MSCs infused at 1 × 106 cells via the tail vein into rats with CCl4-induced cirrhosis improved liver structure. When our unengineered HUCB-MSCs and hHGF-expressing HUCB-MSCs cells were injected at 2 × 106 in CCl4-induced cirrhotic rats, histopathology showed marked improvement in the unengineered HUCB-MSC-treated group, and significant suppression of the fibrotic lesions was observed in the hHGF-expressing HUCB-MSC-treated group. In contrast, large fibrotic areas were seen in liver sections from the PBS-treated group (Figure 6A).
These results clearly indicate that the transplantation of HUCB-MSCs have a therapeutic effect, and that the transplantation of hHGF-expressing HUCB-MSCs has a synergistic regenerative effect on fibrotic livers. Regeneration may be due to the promotion of MSC migration and incorporation into liver tissue, as well as the acceleration of hepatocyte growth by the HGF expressed by HUCB-MSCs. In this context, we also measured the levels of liver enzymes such as ALT, AST and ALP in serum 4 weeks after the initiation of cell therapy, and found that their levels were significantly decreased in both the unengineered HUCB-MSC-treated and hHGF-HUCB-MSC-treated groups. Interestingly, the AST, AST and ALP levels decreased to normal levels in the hHGF-HUCB-MSC-treated group, whereas they were slightly higher than normal in the unengineered HUCB-MSC-treated group.
There are two major categories of liver enzymes used to assess liver disease, leakage enzymes and cholestatic enzymes. Leakage enzymes, such as ALT and AST, leak into the plasma when hepatocyte injury or death occurs. Therefore, high levels of these enzymes in the serum are an indication of hepatocellular injury. Cholestatic enzymes are enzymes for which synthesis in increased as a result of bile retention or the administration of drugs. In the current study, the levels of all three liver enzymes increased in the animals with CCl4-induced liver fibrosis. However, in the HUCB-MSC and HGF-HUCB-MSC-treated groups, these liver enzyme levels decreased back to almost normal levels 4 weeks after stem cell administration, which indicates that HUCB-MSC transplantation can reduce the severity of fibrosis, and hHGF-HUCB-MSCs can enhance liver regeneration better than unengineered HUCB-MSCs.
The data conclusively show that HGF-overexpressing HUCB-MSCs contribute to fibrotic liver regeneration. This approach using genetically modified HUCB-MSCs overexpressing HGF may offer better outcomes for patients with liver fibrosis or cirrhosis.
Acknowledgements and funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A2007693). We wish to thank the Research Institute for Veterinary Science, Seoul National University and the BK21 Program for Veterinary Science 2011.
Author contribution
The amount that authors contributed to this paper was in the following order (most to least): Kyoung-Won Seo, Suh-Young Sohn, Myeong-Jin Nam and Dong-Ha Bhang. Hee-Woo Lee and Hwa-Young Youn are the co-corresponding author.