Proteinase-activated receptor 2 modulates corticotropin releasing hormone-induced brain-derived neurotrophic factor release from microglial cells
Abstract
Brain-derived neurotrophic factor (BDNF) plays a critical role in the pathogenesis of neuropathic pain, but its regulation of BDNF release is not fully understood. To further understand the regulation of BDNF release, the microglial cell line, C8-D1A (microglia, in short), were cultured as a model. The levels of BDNF were determined by enzyme-linked immunoassay. Apoptotic microglia were assessed by flow cytometry. The protease-activated receptor 2 (PAR2) was activated by tryptase. Exposure to corticotripin releasing hormone (CRH) induced BDNF release from microglia. Apoptosis was evident in microglia after activation by CRH. Tryptase-induced PAR2 activation reduced the frequency of apoptosis of microglia, but enhanced the BDNF levels in the culture medium, which was partially blocked by PAR2 antagonists. We conclude that PAR2 agonists can promote the BDNF release from microglia; the PAR2 antagonists may be a potential therapeutic target to attenuate the BDNF-related neuropathic pain.
Introduction
Characterisation of pain is important in the diagnosis and treatment of pain (Konig et al., 2005). Clinically, the symptoms of the underlying disease associating psychological, cognitive and social aspects of the illness, as well as systemic reactions, confound the characterisation of pain (Drewes et al., 2003). Neuropathy arising because of injury to a nerve caused by trauma, infection or pathology is characterised by pain that may be sustained for a long time after the initiating event has healed (Scholz and Woolf, 2002). However, a body of growing evidence indicates that microglia also play an important role in the initiation and persistence of the neuropathic pain (Milligan, 2009). Microglia have immune regulatory functions and play a critical role in neural inflammation in the central nervous system (Tansey, 2010; Reitz et al., 2011). They are involved in the pathogenesis of several neural diseases, such as Alzheimer's and Parkinson's disease, featured as a sustained, chronic neuroinflammatory process (Tansey, 2010; Lilja et al., 2013). Neuroinflammation with microglia involvement is called microgliosis, which includes changes in morphology and proliferation, and modifications in the synthesis and secretion of inflammatory cytokines (Kettenmann et al., 2011).
One of the cytokines produced by microglia is the brain-derived neurotropin factor (BDNF), which is regarded as a pro-survival factor involved in microglial activation in vivo (Jiang et al., 2011). Microgliosis is an important factor in neuropathic pain of the process of neuroinflammation (Zhou et al., 2011). Therefore, BDNF emerges as a potential candidate for mediating neuroinflammation, and the regulation of its release from microglia requires further investigation.
Corticotropin releasing hormone (CRH) is a 41-amino acid neuropeptide, synthesised in the hypothalamus, regulating the hypothalamus-pituitary-adrenal axis (Maras and Baram, 2012). CRH expression and biological function are mediated by its membrane receptors, CRH-R1 and CRH-R2 (Grammatopoulos, 2012). CRH is involved in the pathogenesis of a number of inflammatory disorders, such as allergic diseases (Vasiadi et al., 2012), irritable bowel syndrome (Sagami et al., 2004) and some pain syndromes (La et al., 2008). CRH can also activate microglia in the process of neuropathology (Wang et al., 2007), but the underlying mechanism is not fully understood.
We report here that mouse microglia express corticotropin releasing hormone receptor 1 (CRH R1). Upon exposure to CRH, microglia release BDNF, but activation of protease-activated receptor 2 (PAR2) blocked CRH-induced microglia apoptosis and enhanced BDNF release.
Materials and methods
Reagents
Microglial cell line, C8-D1A cells was purchased from CITech (Shanghai, China). PAR2 antagonist GB-83 was obtained from Axon Medchem (Groningen, The Netherlands). Antibodies of CRH R1 and CRH R2 were purchased from Santa Cruz Biotech (Shanghai, China). BDNF ELISA kit (sensitive limit: 2 pg/ml) was purchased from Shanghai Jianglai Biotech (Shanghai, China). Reagents for real time RT-PCR and Western blotting were purchased from Invitrogen (Shanghai, China). Annexin V kit was purchased from Sigma–Aldrich (Shanghai, China).
Cell culture
Microglia were cultured at 37°C in air plus 5% CO2 in fully humidified atmosphere in DMEM supplemented with 10% fetal bovine serum, 17 mM HEPES, 2 mM L-glutamine and 0.4% penicillin-streptomycin.
Assessment of cell viability
The viability of the cultured microglial cells was assessed by the trypan exclusion assay. The viability of the cells was >95% before use in experiments.
Quantitative real time RT-PCR (qRT-PCR)
Microglia were collected at the end of experiments for RNA extraction with Tryzol reagents. cDNA was synthesised with a reverse transcription kit. qPCR involved a MiniOpticon real-time PCR system with the SYBR green supermix reagents. The 2ΔΔCt method was used calculate the target mRNA levels that were expressed as the percentage of the internal control, β-actin. The primers using in this study include: CRH R1, forward: gaaaccctgcagcagtttga; reverse: ccaaggcatcgctaacactc (NCBI: NM_007762). CRH R2, forward: aggcagcttctccatccttt; reverse: tggtgtgagatgcatccctt (NCBI: NM_009953).
Western blotting
Total proteins were extracted from the collected microglia, separated with sodium dodecyl sulfate polyacrylamide gel electrophoresis, and transferred onto a nitrocellulose membrane. After blocking with 5% skimmed milk for 30 min, the membrane was incubated with the primary antibodies (200 ng/ml) for 1 h at room temperature, followed by incubation with the secondary antibodies for another 1 h at room temperature. Washing with Tris buffered saline supplemented with Triton X-100 (TBST) was performed after each incubation of antibodies. The immune complex was developed with enhanced chemiluminescence. The results were recorded on X-ray films.
Detection of apoptotic microglia
Microglia were stained with propidium iodide (PI; 5 μg/ml for 15 min) and annexin V reagent in a kit, and the cells analysed by flow cytometry using Flowjo software (Tree Star, Inc. Ashland, OR). PI+ cells were dead cells athat were initially gated out. Annexin V+ cells were regarded as apoptotic cells and their frequency was represented as a percentage of the total cells.
Enzyme-linked immunoassay (ELISA)
BDNF levels were determined by ELISA with a commercial reagent.
Statistics
The data are presented as mean ± SD. Differences between groups were analysed with Student's t-test, with P < 0.05 being set as a significant difference.
Results
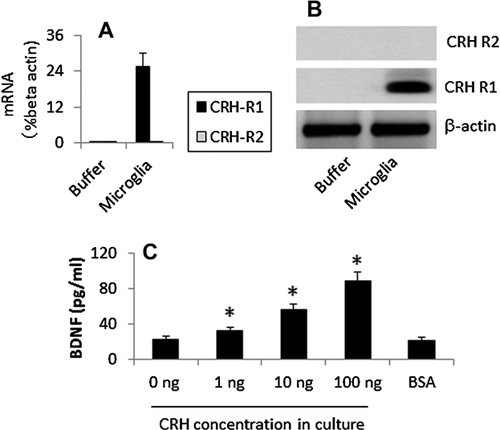
Activation of TLR4 increases CRH receptor expression in microglia
CRH triggers microglia to release BDNF
Isolated microglia expressed the CRH R1, but not CRH R2. Exposure to CRH induced the release of BDNF from microglia in a dose-dependent manner (Figure 1).
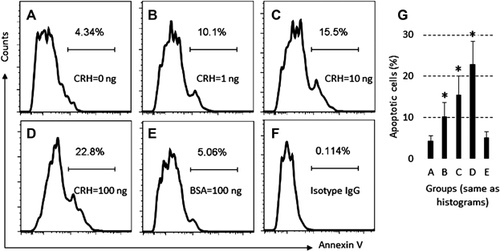
Exposure to CRH induces microglia apoptosis
To test whether T cells become apoptosis after activation (Chhabra, 2010), we treated microglia with CRH first, which were cultured for 48 h. The microglia were stained with propidium iodide (PI) and annexin V reagents. Flow cytometry shows that exposure to CRH markedly increases the apoptotic rate of microglia (Figure 2).
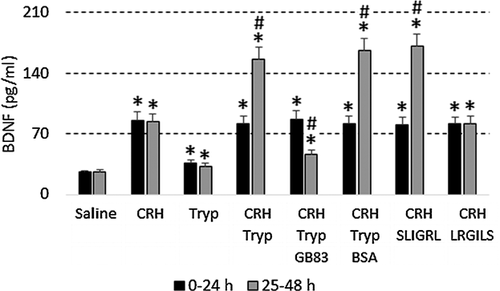
Activation of PAR2 decreases the rate of apoptosis microglia
Since activation of PAR2 attenuates apoptosis (Peng et al., 2013) and microglia express PAR2 (Zhang et al., 2012), we checked whether activation of PAR2 could attenuate microglial apoptosis after exposure to CRH. The results showed that microglia expressed PAR2 (data not shown). Microglia were then to exposed CRH or/and tryptase in tculture. Compared to exposure to either CRH or tryptase alone, the combination of the two caused markedly less apoptosis. To confirm the effect of PAR2 in the process, we added the PAR2 antagonist, GB83 (2 µM), to the culture and found that attenuation of apoptosis of microglia was abolished. In other experiments, we added active PAR2 peptide, SLIGRL, to the culture medium; this, but not LRGILS, also markedly inhibited CRH-induced microglia apoptosis (Figure 3).

Activation of PAR2 enhances CRH-induced BDNF release from microglia
In assessing the levels of BDNF in the culture medium (Figure 3), we found that, in a similar manner to Figure 2, exposure to CRH increased the BDNF in the culture, which was enhanced when exposure was to both CRH and tryptase (or the PAR2 active peptide SLIGRL). Blocking PAR2 with GB83 abolished the increase in BDNF levels caused by tryptase (Figure 4).
Discussion
Microglia express CRH R1 and PAR, and exposure to CRH induces microglia to release BDNF as well as inducing apoptosis; this can be prevented by adding tryptase or active PAR2 peptides.
The data indicate that microglia can be activated by CRH, causing the release of BDNF. Others have shown that microglia express CRH receptors, and that the CRH analogue, urotropin, can activate microglia to modulate microbial product-induced microglia activities (Wang et al., 2007). Xiao et al. (2011) indicate that an interaction between CRHR1 and BDNF genes constitutes susceptibility to recurrent major depressive disorder. Xu et al. (2000) found that CRH and BDNF are associated with chronic pain in a rat model. BDNF has multiple functions, such as involving triggering pain (Xiao et al., 2011), promoting local neurogenesis, and eliciting anxiolytic-like activities (Quesseveur et al., 2013).
Activation induced cell death has been described in immune cell death; for example after activation by specific antigens, antigen specific T cells die through the apoptotic pathway soon after the exposure to specific antigens (Zhang et al., 2004). Another term relating to activation-induced apoptosis was also coined (Pender, 1999). Such a phenomenon not only occurs in T cells, but in other cell types, for example dendritic cells (Pender, 1999). Our data indicate that this also happens in microglia. After exposure to CRH, the microglia release BDNF, a sign of activation. These microglia soon became apoptotic in line with the concept of activation-induced cell death.
Our data show that microglia express PAR2. Exposure to both CRH and tryptase attenuated microglia apoptosis. Tryptase, a major cytokine of mast cells, represents ∼25% of the total protein of mast cells. Upon activation, these cells release cytokines, including tryptase, which is involved in a number of inflammatory processes. However, CRH can be released by a number of other factors, such as under psychological stress. Therefore, the increases in CRH and tryptase may occur concurrently in the same region of the brain tissue. From the present data, it can be reasoned that concurrent exposure to CRH and tryptase can increase BDNF release and reduce microglia apoptosis, thereby extending the length of BDNF release.
In summary, the data indicate that microglia express CRH R1 and PAR2. Exposure to CRH induces BDNF release and the microglia apoptosis. Concurrent exposure to CRH and PAR2 activator, tryptase, or the PAR2 active peptide, can reduce the rate of apoptotic microglia and extend the BDNF release period.