Hypoxia induces adipocyte differentiation of adipose-derived stem cells by triggering reactive oxygen species generation
Abstract
Generation of reactive oxygen species (ROS) by NADPH oxidase 4 (Nox4) induces the proliferation and migration of adipose-derived stem cells (ASCs). However, the functional role of mitochondrial ROS (mtROS) generation in ASCs is unknown. Therefore, we have investigated whether hypoxia induces the differentiation of ASCs via ROS generation. We also have tried to identify the cellular mechanisms of ROS generation underlying adipocyte differentiation. Hypoxia (2%) and ROS generators, such as antimycin and rotenone, induced adipocyte differentiation, which was attenuated by an ROS scavenger. Although Nox4 generates ROS and regulates proliferation of ASCs, Nox4 inhibition or Nox4 silencing did not inhibit adipocyte differentiation; indeed fluorescence intensity of mito-SOX increased in hypoxia, and treatment with mito-CP, a mtROS scavenger, significantly reduced hypoxia-induced adipocyte differentiation. Phosphorylation of Akt and mTOR was induced by hypoxia, while inhibition of these molecules prevented adipocyte differentiation. Thus hypoxia induces adipocyte differentiation by mtROS generation, and the PI3K/Akt/mTOR pathway is involved.
Introduction
Adipose-derived stem cells (ASCs) are a possible substitute for other types of stem cells for clinical applications in tissue repair and regeneration (Parker and Katz, 2006; Mizuno and Nambu, 2011; Philips et al., 2012). We have recently shown that hypoxia preconditioning significantly increases the regenerative potential of ASCs (Lee et al., 2009; Kim et al., 2009b). Likewise, the beneficial effects of hypoxia on ASCs have been reported in various experimental systems (Chung et al., 2009; Lee et al., 2009; Kim et al., 2011, 2012). By contrast, Trayhurn et al. (2009) found that hypoxia increases the production of inflammatory adipokines implicated in the pathogenesis of obesity and related metabolic diseases. In our investigations into the key factor(s) stimulated during hypoxia, we found that reactive oxygen species (ROS) produced by NADPH oxidase (Nox) chiefly mediate the proliferation and migration of ASCs (Kim et al., 2011, 2012). Hypoxia significantly induced MAPK and PI3K/Akt pathways in ASCs. However, the effect of hypoxia on adipocyte differentiation and the underlying mechanism of adipogenesis have not been fully characterized.
Hypoxia influences cellular functions, including metabolism, survival, proliferation, migration, and adipocyte differentiation. All of these cellular functions are directly or indirectly controlled by free radicals, such as ROS or reactive nitrogen species (RNS). In a preliminary study, we found that expression level of nitric oxide (NO) synthase is low in ASCs, and a stimulatory effect of RNS donors is insufficient to increase the proliferation and migration of ASCs. However, there are controversial reports on whether NO-cyclic GMP (cGMP) signals are involved in the growth and differentiation of stem cells (Mujoo et al., 2011; Fuseler and Valarmathi, 2012). For example, NO donors at high concentration and downstream second messenger cGMP induce adipogenic and angiogenic differentiation of ASCs (Bassaneze et al., 2010; Hemmrich et al., 2010). Therefore, it is important to address which kinds of free radicals mediate adipocyte differentiation of ASCs.
ROS are generated by a variety of extracellular and intracellular mechanisms (Shimomura et al., 2006), but Nox is the major source of ROS generation in cells. Other sources of ROS are cyclooxygenase, lipoxygenase, xanthine oxygenase, and cytochrome P-450 mono-oxygenase. Of them, ROS generation by Nox4 induces proliferation and migration of ASCs (Kim et al., 2011, 2012). However, the functional role of mitochondrial ROS (mtROS) generation in ASCs is not fully understood. Therefore, we have investigated whether hypoxia induces adipocyte differentiation of ASCs via ROS generation, and tried to identify the cellular mechanisms of ROS generation that mediate adipocyte differentiation of ASCs.
Materials and methods
Materials
N-Acetyl-L-cysteine (NAC), dibenziodolium chloride (DPI), N-nitro-L-arginine methyl ester hydrochloride (NAME), triphenylphosphine (TPP), antimycin and rotenone were obtained from Sigma (St. Louis, MO). S-Nitroso-N-acetyl-D,L-penicillamine (SNAP) and S-nitroso-L-glutathione (GSNO) were purchased from Cayman Chemical (Ann Arber, MI).
Synthesis of mito-carboxy proxyl
Mito-carboxy proxyl (CP) was synthesized by following the procedure of Kalyanaraman (Dhanasekaran et al., 2005) and Boelsterli (Lim et al., 2008).
Cell culture and inhibition study
Sampling of human subcutaneous adipose tissue and isolation of ASCs was done as before (Kim et al., 2007, 2009a). ASCs were characterized by trans-differentiation and analysis of cell surface markers by flow cytometry. ASCs were grown in Minimum Essential Medium-alpha (Hyclone, Thermo Scientific, Logan, UT) with 10% fetal bovine serum (GIBCO), 1% penicillin and streptomycin (GIBCO) at 37°C in a humidified air plus 5% CO2 (normoxia), or 5% CO2, 2% O2 and balanced N2 (hypoxia). Antimycin (10 nM), rotenone (10 pM), SNAP (10 nM) and GSNO (10 nM) were used in the functional study. For the inhibition of ROS generation, 0.1 or 1 mM NAC, 1 μM DPI, 20 mM Nox4 siRNA transfection, and 2–10 μM mito-CP were used. For the inhibition of RNS generation, 0.1 mM NAME were used under hypoxia.
ROS or RNS generation assay
ROS generation was measured using 2′,7′-dichlorofluorescin diacetate probe (DCF-DA, Molecular Probes, Eugene, OR), and RNS generation was measured using diaminofluorescein-2diacetate (DAF-DA, Sigma). ASCs (4 × 105 cells) were seeded into 60 mm culture dishes. They were pretreated with DCF-DA or DAF-DA (10 μM) for 20 min and incubated under hypoxia for 20 min at 37°C in the dark place before being harvested using trypsin-EDTA. Fluorescence intensity of DAF-DA or DAF-DA was measured and quantified by flow cytometry.
Adipocyte differentiation
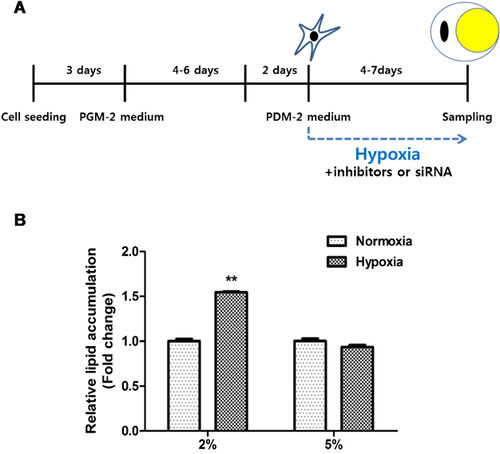
ASCs (4 × 104) were seeded in 12-well plates in complete medium. When they had become confluent, the medium was replaced with preadipocyte growth medium-2 (PGM-2, Lonza, Walkersville, MD) for 4–6 days. They were then treated with hypoxia, ROS generators, or RNS generators in preadipocyte differentiation medium-2 (PDM-2, Lonza) for 4–7 days (Figure 1A). Adipocyte differentiation was confirmed by using oil-red-O staining and RT-PCR.
Oil red-O staining
ASCs were washed with PBS and fixed with 10% formalin for 1 h. After fixing, cells were washed with PBS and stained with oil red-O solution for 2 h. This solution was made of 60% oil red-O stock solution (1% oil red-O powder in isopropanol) in distilled water. The stained cells were washed twice with distilled water and examined microscopically. For the quantification of oil red-O, cells were incubated with isopropanol for 30 min, and the color in the supernatant of the decolorized cells was measured by the absorbance at 492 nm with a micro-plate reader (TECAN).
RT-PCR
Total cellular RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA) followed by a reverse transcription with cDNA synthesis kit (Promega, Madison, WI). cDNA was synthesized from 1 µg total RNA using 200 U of reverse transcriptase (M-MLV RT) and 50 ng/μl oligo(dT). Primers were used as follows; PPARγ, forward: 5′-GCGGAGATCTCCAGTGATATC-3′ and reverse: 5′-TCAGCGACTGGGACTTTTCT-3′, C/EBPβ, forward: 5′-GAATCTCCTAGTCCTGGCTC-3′ and reverse: 5′-GATGAGAACAGAACGAGTAC-3′, and GAPDH, forward: 5′-CGAGATCCCTCCAAAATCAA-3′ and reverse: 5′-TGTGGTCATGAGTCCTTCCA-3′. PCRs were performed in a final volume of 25 µl reaction mixture that contained 2 µl of the RT reaction mixture, 15 mM MgCl2, 1.25 mM dNTP, 20 pM of each primer and 0.5 U of Taq polymerase (Promega). Thermal cycling over 40 cycles consisted of an initial denaturation at 94°C for 5 min, 94°C for 30 s, 56°C for 30 s, 72°C for 30 s, terminated by a final extension at 72°C for 5 min. GAPDH mRNA level was used for sample standardization. After electrophoresis on 1.5% agarose gel, each band was quantified by densitometer (Bio-Rad Laboratories, Hercules, CA).
Phosphokinase chip array
For phosphokinase chip array, ASCs were seeded into serum-free medium in 100 mm dishes. The following day, ASCs were incubated in hypoxia for 10 min. Cells were harvested by lysis buffer (R&D Systems) and proteins were isolated. Phosphorylation of signaling molecules was detected with a phosphokinase array kit (R&D Systems).
Statistical analysis
Data are representative of triplicate independent experiments. The statistical significance of the differences among groups was tested using student's t-test. P < 0.01 was considered to be significant.
Results
Hypoxia at 2% oxygen increased adipocyte differentiation
In a pilot study, ASCs were fully differentiated into adipocytes 2–3 weeks after addition of adipocyte differentiation medium. However, we usually measured the early induction of adipocyte differentiation at 4 and 7. Compared with normoxia and 5% hypoxia, 2% hypoxia significantly increased lipid accumulation of ASCs (Figure 1B). mRNA expression of adipocyte differentiation markers, such as peroxisome proliferator-activated receptor-γ (PPARγ) and CCAAT/enhancer-binding protein-β (C/EBPβ), were upregulated in 2% hypoxia (data not shown).
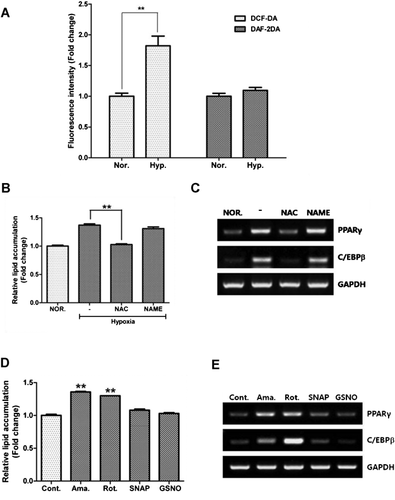
ROS, but not RNS, generation induced adipocyte differentiation
Flow cytometry was used to measure the free radicals generated by hypoxia. The geometric mean of DCF-DA, a ROS indicator, significantly increased under hypoxia, while that of DAF-DA, a RNS indicator, was unaltered (Figure 2A, P < 0.01). By scavenging every free radical using NAC (a ROS scavenger) and NAME (a RNS scavenger), the former, but not NAME, significantly attenuated hypoxia-induced lipid accumulation (Figure 2B, P < 0.01). Likewise, hypoxia-induced mRNA expression of PPAR-γ and C/EBP-β was not downregulated by NAME, but by NAC treatment (Figure 2C). ROS generators such as antimycin (10 nM) and rotenone (10 pM) induced lipid accumulation (Figure 2D) and upregulated mRNA expression of PPAR-γ and C/EBP-β expression (Figure 2E), but RNS generators such as SNAP (10 nM) and GSNO (10 nM) did not. This suggests that hypoxia increases adipocyte differentiation of ASCs mainly via ROS generation.
Nox4-produced ROS does not induce adipocyte differentiation
We previously demonstrated that Nox4-produced ROS results in an increase in the proliferation and migration of ASCs. Here, we investigated whether or not Nox4 can induce an increase in adipocyte differentiation. However, a pharmacological inhibitor of Nox4 (DPI, 100 nM) or siRNA transfection of Nox4 did not reduce lipid accumulation (Supplemental Figure 1A and 1C, respectively), nor downregulation of PPAR-γ and C/EBP-β expression (Supplemental Figure 1B and 1D, respectively), thus indicating that Nox-induced ROS generation does not mediate adipocyte differentiation.
Hypoxia increases mtROS generation
Since mitochondrial respiration is major ROS source, we investigated whether mtROS generation under hypoxia induces adipocyte differentiation. mtROS generation was assessed by measuring the fluorescence intensity of mito-SOX in a flow cytometry. Hypoxia significantly increased the fluorescence intensity of mito-SOX in ASCs (Figure 3A).
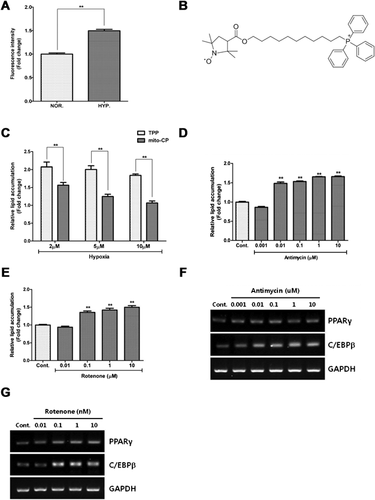
mtROS generation induced adipocyte differentiation
Mito-CP (Figure 3B), a mitochondria specific ROS scavenger, was synthesized to investigate the involvement of mtROS generation in adipocyte differentiation. Compared to TPP treatment (as negative control), scavenging mtROS with mito-CP significantly reduced hypoxia-induced lipid accumulation in a dose-dependent manner (Figure 3C, P < 0.01).
Antimycin and rotenone induce ROS generation by inhibiting mitochondrial respiratory complex III and complex I, respectively (Li et al., 2003; Rieske et al., 1967). We therefore examined if these mtROS generators induce adipocyte differentiation. Different concentrations of antimycin (Figure 3D, P < 0.01) and rotenone (Figure 3E, P < 0.01) significantly induced lipid accumulation in a concentration dependent manner. Antimycin (Figure 3F) and rotenone (Figure 3G) also induced the PPAR-γ and C/EBP-β expression in ASCs.
Akt and mTOR phosphorylation are involved in adipocyte differentiation
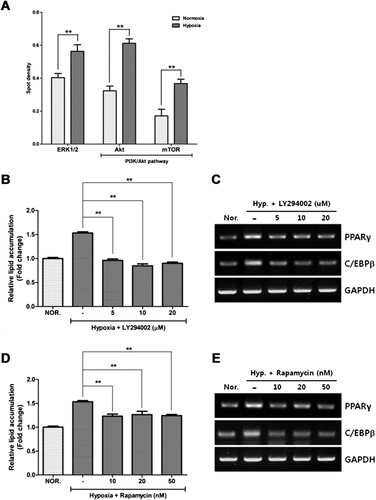
Phosphokinase antibody array was carried out to investigate the signaling pathways activated by hypoxia (Figure 4A). Phosphorylation of ERK1/2, Akt, and mTOR was significantly increased by hypoxia (P < 0.01), which had been previously demonstrated by Western blot analysis (Kim et al., 2011). To see whether the PI3K/Akt/mTOR and MAPK pathways are involved in adipocyte differentiation, we found that the inhibitors of PI3K/Akt (LY294002, Figure 4B) and mTOR (rapamycin, Figure 4C) significantly reduced lipid accumulation in ASCs. LY294002 (Figure 4D) and rapamycin (Figure 4E) reduced the PPAR-γ and C/EBP-β expression in ASCs. However, U0126, an inhibitor of the MAPK pathway, did not reduce the adipocyte differentiation (Supplemental Fig. 2). The findings indicate that PI3K/Akt/mTOR pathway plays a pivotal regulatory role in adipocyte differentiation.
Discussion
The reported data should shed some light on important aspects of hypoxia-induced adipocyte differentiation of ASCs, as well as identify the cellular mechanisms of ROS-mediated adipocyte differentiation. Hypoxia (2% oxygen) induced adipocyte differentiation via ROS, but not RNS, generation. Furthermore, Nox4 inhibition or silencing did not inhibit adipocyte differentiation. However, hypoxia induced mtROS generation, and mito-CP (mtROS scavenger) treatment significantly reduced hypoxia-induced adipocyte differentiation. Hypoxia led to phosphorylation of Akt and mTOR, and inhibition of the PI3K/Akt/mTOR pathway inhibited adipocyte differentiation. Thus hypoxia induces adipocyte differentiation by mtROS generation, and the PI3K/Akt/mTOR pathway is involved in adipocyte differentiation of ASCs.
Adipogenesis is triggered by signaling molecules that induce conversion of ASCs to preadipocytes that further differentiate into adipocytes (Cristancho and Lazar, 2011). The BMP and Wnt families are key mediators involved in early differentiation of ASCs into preadipocytes, while insulin-like growth factor 1 (IGF1), glucocorticoid, and cyclic AMP (cAMP) trigger differentiation of preadipocytes into adipocytes (Tang and Lane, 2012). In addition to these signaling molecules, we have shown that hypoxia-induced ROS generation contributes to adipocyte differentiation of ASCs, thereby accelerating the early differentiation of ASCs into preadipocytes.
In general, ROS donors induce adipocyte differentiation of mesenchymal stem cells, while antioxidant compounds inhibit adipocyte differentiation (Calzadilla et al., 2011; Kanda et al., 2011; Tormos et al., 2011; Younce and Kolattukudy, 2012). However, the role of Nox4-induced ROS generation in adipocyte differentiation remains under debate. For example, Higuchi et al. (2013) showed that Nox4 overexpression induced the adipocyte differentiation of ASCs, while Li et al. (2012) demonstrated that Nox4 deficiency or knock-down induced obesity and adipocyte differentiation. However, based on the present results, we can conclude that mtROS generation, but not Nox4, is involved in adipocyte differentiation during hypoxia. This is because mtROS generators such as antimycin and rotenone induce adipocyte differentiation, and treatment with mtROS scavengers during hypoxia inhibited it (Figure 3). Nox4 silencing also did not attenuate the hypoxia-induced adipocyte differentiation, but slightly accelerated it (supplemental Figure 1).
The effect of NO donors on the proliferation and adipocyte differentiation of ASCs remains under controversy. For instance, Hemmrich et al. (2010) reported that diethylenetriamine (a NO generator at 30–150 μM) enhances the adipogenic differentiation and inhibits the proliferation of ASCs. However, we found low concentrations of SNAP (1–10,000 nM) and GSNO (1–10,000 nM) had little or no effect on the proliferation and adipogenic differentiation of ASCs. This discrepancy may result from differences in the NO donors or the concentrations used. However, considering that a low concentration of ROS generator (below 10 nM) could increase the proliferation and differentiation of ASCs, ROS appears to be superior to NO donors in inducing proliferation and differentiation of ASCs.
We have shown that hypoxia induces adipocyte differentiation via ROS, but not RNS generation. Hypoxia also induces mtROS generation, while mito-CP, a mtROS scavenger, treatment significantly reduces hypoxia-induced adipocyte differentiation. Hypoxic conditions result in the phosphorylation of Akt and mTOR, and inhibition of the PI3K/Akt and mTOR pathways inhibits adipocyte differentiation. In summary, hypoxia induces adipocyte differentiation by generation of mtROS, which is mediated by the PI3K/Akt/mTOR pathway.
Acknowledgements and funding
This study was primarily supported by a grant of basic Science Research Program through the National Research Foundation of Korea (2011-0019636, 2011-0007751, and 2012-003803). YX was supported by NIH (HD-034852, AT-004422). The authors declare no competing financial interests.
Conflict of interest statement
None declared.