Mental retardation-related protease, motopsin (prss12), binds to the BRICHOS domain of the integral membrane protein 2a
Abstract
Motopsin (prss12), a mosaic serine protease secreted by neuronal cells, is believed to be important for cognitive function, as the loss of its function causes severe nonsyndromic mental retardation. To understand the molecular role of motopsin, we identified the integral membrane protein 2a (Itm2a) as a motopsin-interacting protein using a yeast two-hybrid system. A pull-down assay showed that the BRICHOS domain of Itm2a was essential for this interaction. Motopsin and Itm2a co-localized in COS cells and in cultured neurons when transiently expressed in these cells. Both proteins were co-immunoprecipitated from lysates of these transfected COS cells. Itm2a was strongly detected in a brain lysate prepared between postnatal day 0 and 10, during which period motopsin protein was also enriched in the brain. Immunohistochemistry detected Itm2a as patchy spots along endothelial cells of brain capillaries (which also expressed myosin II regulatory light chain [RLC]), and on glial fibrillary acidic protein (GFAP)-positive processes in the developing cerebral cortex. The data raise the possibility that secreted motopsin interacts with endothelial cells in the developing brain.
Abbreviations
-
- Itm
-
- integral membrane protein
-
- GFAP
-
- glial fibrillary acidic protein
-
- PBS
-
- phosphate-buffered saline
-
- PCR
-
- polymerase chain reaction
-
- RLC
-
- regulatory light chain
Introduction
Motopsin (prss12), also known as neurotrypsin, is a serine protease secreted by neuronal cells in brain regions including the hippocampus, cerebral cortex, and cranial nerve nuclei (Yamamura et al., 1997; Mitsui et al., 2007). This protease is important for neuronal function because the loss of motopsin function causes severe nonsyndromic mental retardation in humans (Molinari et al., 2002) and enhanced social behavior in mice (Mitsui et al., 2009). Although axonal injury of facial nerves transiently reduces the expression levels of motopsin mRNA, the recovery of neuronal function accompanies the restoration of the expression of motopsin mRNA, which suggests the involvement of motopsin in neuronal plasticity (Numajiri et al., 2006; Mitsui et al., 2008). Overexpression of motopsin in cultured neurons shows that depolarization causes the exocytosis of motopsin from presynaptic vesicles (Frischknecht et al., 2008). The extracellular matrix protein, agrin, is a possible substrate for motopsin (Reif et al., 2007; Stephan et al., 2008), and agrin may be important for the formation and maintenance of excitatory synapses (Hilgenberg et al., 2006; Ksiazek et al., 2007). In fact, motopsin deficiency disturbs filopodia formation induced by long-term potentiation and decreases the number of a postsynaptic structure, spine, on hippocampal neurons (Mitsui et al., 2009; Matsumoto-Miyai et al., 2009). These reports suggest that motopsin modulates neuronal plasticity by modifying agrin function.
In contrast, motopsin is detected in the dendrites or the somatic body of neurons under normal expression levels (Mitsui et al., 2007). The expression of motopsin mRNA in the cerebral cortex culminates around the second week after birth (Iijima et al., 1997; Wolfer et al., 2001), then gradually decreases through the lifetime of an individual. This temporal expression pattern raises the additional possibility that motopsin may be involved in the development of the cerebral cortex. The mosaic structure of motopsin, which consists of a signal sequence at the N-terminus followed by a proline-rich domain, a kringle domain, three scavenger receptor cysteine-rich domains, and a protease domain at the C-terminus, suggests that motopsin interacts with other molecules. Motopsin secreted at synaptic clefts appears to be captured around the synapses (Frischknecht et al., 2008). To identify motopsin-interacting proteins, we screened a mouse embryonic cDNA library using a yeast two-hybrid system, and found that Itm2a bound to motopsin through its BRICHOS domain and that Itm2a was highly expressed in the cerebral cortex during the first 2 weeks of life, a period during which motopsin is highly produced in cerebral neurons.
Materials and methods
Cell lines
The African green monkey kidney fibroblast-like cell line, COS1 (ATCC No. CRL-1650), was purchased from DS Pharma Biomedical Co., Ltd (Osaka, Japan). COS1 cells were cultured in Dulbecco's modified Eagle's medium (Life Technologies Co.) containing 7% fetal bovine serum at 37°C in air plus 5% CO2.
Animals
Pregnant C57/BL6NCrSlc mice were purchased from Japan SLC, Inc. (Shizuoka, Japan) and kept in a standard cage in a 12-h light/dark cycle with access to food and water ad libitum. For the preparation of hippocampal neurons, mice were sacrificed at 17.5 days postcoitum and fetus were recovered. For Western blot analysis of Itm2a expression, pups were killed at the indicated age and brains were prepared as previously described (Osaki et al., 2011). All animal experiments were performed in accordance with Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology, Japan and approved by the Animal Experiment Committee at Gunma University and Kochi University.
Yeast two-hybrid screening
A yeast two-hybrid screen was done with the MATCHMAKER Gal4 Two-Hybrid System 3 (Clontech, Mountain View, CA), as reported earlier (Mitsui et al., 2013). As protease activity is sometimes toxic to host cells, the Ser711 residue essential for the proteolytic activity of motopsin was replaced with Ala residues (S711A). Two polymerase chain reaction (PCR) fragments were amplified using the following primer sets: primer 1 (5′-CAACTCGAGGAGCTATGCAG-3′) and primer 2 (5′-TGGTCCTCCAGCGTCTCCCTGG-3′), or primer 3 (5′-CCAGGGAGACGCTGGAGGACCA-3′) and primer 4 (5′-GCGAATTCCATAAGTTACAGACTGGTGACACTT-3′). The sequences that code for the substituted Ala residue are underlined. The annealing of two PCR fragments between primers 2 and 3 recovered a 604-bp fragment, which was amplified using primers 1 and 4. The 604-bp fragment was inserted into the pBluescript/motopsin vector, between the XhoI and EcoRI restriction sites. The nucleotide sequence of the resulting construct was confirmed using an automatic sequencer (ABI310 sequencer, Applied Biosystems, Foster City, CA). To construct a bait vector, a cDNA fragment corresponding to motopsin S711A was amplified using primer 5 (5′-GCCGAATTCGATCCGGTCTCGCGCTCTCC-3′) and primer 4, and was inserted into the EcoRI restriction site of the pGBKT7 vector. Yeast AH109 cells transformed with the bait vector were mated with the Y189 yeast strain, which was pretransformed with a mouse E17 cDNA library, and were plated on SD/–Leu/–His/–Trp/ + 3 amino-1,2,4-triazole medium. Growing colonies were replated onto SD/–Ade/–Leu/–His/–Trp/5-bromo-4-chloro-3-indolyl alpha-D-galactopyranoside medium. Blue colonies were lysed with zymolyase to recover the cDNAs. The inserted cDNAs were amplified using 5′ and 3′ AD LD-Insert Screening Amplimer primers that were supplied in the kit and were directly sequenced using an automated sequencer. The cDNA encoding the full-length open reading frame of Itm2a was amplified from a mouse brain total RNA extract by reverse transcription-PCR using the 5′-CAAGATACTGATTCGAGCCG-3′ and 5′-GTCAGACGGTAAGTTCCTGA-3′ primers and was cloned into the pGEM-T Easy vector (Promega Corp., Madison, WI). The amplified cDNAs were sequenced with a T7 primer using an automatic sequencer.
In vitro interaction between motopsin and Itm2a
The in vitro interaction between Itm2a and motopsin was confirmed by a pull-down assay. A cDNA fragment encoding the extracellular region (Gly70–Glu262) of Itm2a was inserted at the EcoRI site of the pGADT7 vector (Clontech). The HA-tagged extracellular domain of Itm2a was prepared using the pTrcHis B vector (Life Technologies Corp., Carlsbad, CA), as before (Yamaguchi et al., 2002). A lysate from cells expressing the HA-tagged Itm2a extracellular domain was applied onto S-protein agarose bound to NUS-tagged motopsin (Mitsui et al., 2007). Unbound proteins were washed with 0.1% Triton X-100, 20 mM Tris–Cl (pH 7.4), and 150 mM NaCl. Bound proteins were eluted with SDS–polyacrylamide gel electrophoresis (SDS–PAGE) sample buffer and were detected by Western blot using an anti-HA monoclonal antibody (InvivoGen, San Diego, CA) or an anti-(His)6 polyclonal antibody (Bethyl Lab, Inc., Montgomery, TX).
Confirming interaction between motopsin and Itm2a
COS1 cells were cultured in Dulbecco's modified Eagle medium containing 7% fetal calf serum. Mouse hippocampal neurons were prepared from E17.5 embryos, as described by Goslin and Banker (1998), and were cultured in Neurobasal medium containing B27 supplement (Life Technologies Corp.) on a poly-L-lysine-coated glass slide with water-repellent print (Matsunami Glass Ind. Ltd., Osaka, Japan). The cDNA encoding Itm2a was amplified using the primers 5′-TTGCGGCCGCGTGAAGATCGCCTTCAACAC-3′ and 5′-GGGGTACCTATCTGTCACATTTCACTCC-3′ and was inserted between the NotI and KpnI restriction sites of the pCRUZ Myc B vector (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), which expressed Itm2a fused with Myc tag at the amino-terminal (pCRUZ/mouse Itm2a). To express HA-tagged motopsin, a cDNA fragment corresponding to motopsin lacking the termination codon was amplified using high-fidelity Taq polymerase and primers (5′-CCAAGCTTCATGGCGCTCGCCCGCTGCG-3′ and 5′-AGAAGCTTGCAGACTGGTGACACTTTTTATC-3′) and was inserted into the D-HA vector at the HindIII restriction site (D-HA/mouse motopsin). The cultured cells were transfected with D-HA/mouse motopsin and/or pCRUZ/mouse Itm2a using Lipofectamine 2000 (Invitrogen). For immunocytochemistry, cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) at 4°C for 20 min 2 days after transfection. After washing with PBS containing 0.3% Triton X-100 (PBS-T), cells were reacted with the primary antibodies at 4°C for 18 h (1:1,000 anti-motopsin IgG or 1:1,000 anti-Myc monoclonal antibody, clone MC045, Nacalai Tesque, Inc., Kyoto, Japan). Cells were subsequently reacted with anti-rabbit IgG/Alexa Fluor488 and anti-mouse IgG/Alexa Fluor594 (Life Technologies Corp.) for 2 h at room temperature. Slides were mounted with VECTASHIELD (Vector Lab, Burlingame, CA) and analyzed using a fluorescent microscope (Eclipse 80i, Nikon Co., Tokyo, Japan) coupled to a VB-7010 CCD camera with an inbuilt cooling system (Keyence Co., Osaka, Japan). For immunoprecipitation, cells were lysed with ice-cold RIPA buffer (PBS containing 1% NP-40, 0.5% deoxycholate, and 0.1% SDS). Two micrograms of normal rabbit IgG was added to aliquots of the lysate, which were incubated at 4°C for 3 h and recovered using anti-rabbit IgG–agarose (Wako Pure Chemical Industries Ltd., Osaka, Japan). Two micrograms of anti-Myc antibody (Sigma, St. Louis, MO) was incubated with the lysates at 4°C overnight. The immunocomplexes were recovered using anti-rabbit IgG–agarose and eluted with SDS–PAGE sample buffer. The precipitated proteins were detected by Western blot using anti-HA and anti-Myc monoclonal antibodies.
Immunohistochemistry
The distribution of Itm2a in the mouse brain was investigated using anti-mouse Itm2a (Santa Cruz Biotechnology, Inc.), anti-GFAP (clone GA-5, Thermo Fisher Scientific, Inc., Fremont, CA), and anti-myosin II RLC antibodies, as previously described (Mitsui et al., 2007).
Western blot analysis
Mouse brains were homogenized at the indicated age in five volumes of ice-cold extraction buffer (20 mM Tris–Cl [pH 7.5], 1% Triton X-100, 10 mM ethylene-diaminetetraacetic acid) containing protease inhibitors. The homogenate was centrifuged at 10,000g for 15 min and the supernatant was recovered. Fifty micrograms of extracted proteins was separated by SDS–polyacrylamide (10%) gel electrophoresis. After electroblotting onto a polyvinylidine fluoride membrane (Immobilon-P, Millipore, Billerica, MA), the proteins were reacted with goat anti-mouse Itm2a IgG, and with anti-goat IgG antibody conjugated with peroxidase (Santa Cruz Biotechnology, Inc.). The immunoreaction was detected using the ECL Plus Western blotting detection system (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and a LAS-4000 chemiluminescent detector (Fujifilm Co., Tokyo, Japan).
Results
Interaction between motopsin and Itm2a
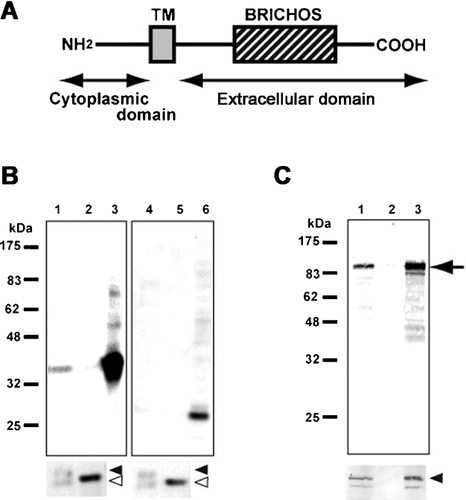
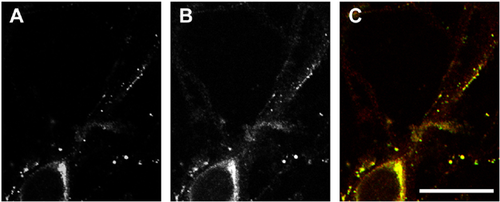
The sequence analysis of 96 clones, which were suggested to encode a motopsin-interacting protein by the two-hybrid screen, showed that 9 of these clones encoded Itm2a (Figure 1A), which is classified as a member of the type II integral membrane protein (Itm2) family. To confirm the interaction between motopsin and Itm2a, a pull-down assay was used. A lysate from cells expressing Itm2a was applied to recombinant motopsin fixed on S-protein agarose. The extracellular domain of Itm2a bound to motopsin-fixed S-protein agarose, but not to Nus-tag-fixed agarose (Figure 1B). Recombinant Itm2a lacking the BRICHOS domain did not bind to motopsin. To confirm this interaction, HA-tagged motopsin and Myc-tagged Itm2a were expressed in COS cells and were co-immunoprecipitated. The anti-Myc antibody recovered motopsin and Myc-tagged Itm2a from a lysate of cells that expressed both proteins; however, the expression of motopsin alone did not lead to the precipitation of either motopsin or Itm2a by the anti-Myc antibody (Figure 1C). Immunocytochemistry using an anti-Myc antibody showed a punctate pattern in the neurites of neuronal cells that expressed Myc-tagged Itm2a (Figure 2). This distribution pattern was similar to that of motopsin. The merged image clearly shows that motopsin and Itm2a co-localized within the speckles. The results of the co-immunoprecipitation assay and the co-localization analysis in cultured neurons suggest the ability of motopsin to bind Itm2a under physiological conditions.
Expression of Itm2a in the developing mouse brain
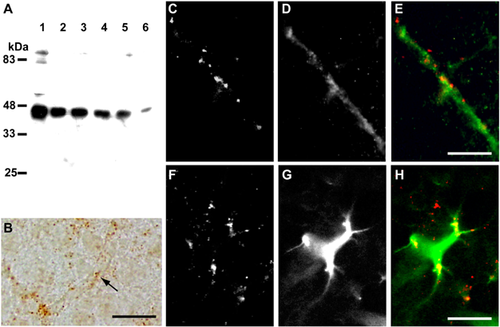
Motopsin is expressed in the cerebral cortex most abundantly around postnatal day (P) 10 and gradually decreases, but remains detectable in the adult brain (Iijima et al., 1997; Mitsui et al., 2007). To address the possibility that motopsin interacts with Itm2a in vivo, the expression of Itm2a was analyzed by Western blot and immunohistochemistry. Itm2a was strongly detected in the lysates of cerebral cortex between P0 and P10, and gradually decreased toward adulthood (Figure 3A), which is an expression pattern similar to that of motopsin (Iijima et al., 1997). In the postnatal brain, Itm2a immunoreactivity (IR) was scattered (as small dots) across the cerebral cortex (Figure 3B). But most of Itm2a IR was found located along capillaries. Double-fluorescent immunohistochemistry indicated that most of the Itm2a IR was observed in cells that expressed myosin II RLC (Figure 3C–E), which is a marker of endothelial cells in brain capillaries (Ishmael et al., 2008). This result is consistent with a report that showed that Itm2a mRNA is enriched in brain microvascular endothelial cells compared with aortic endothelial cells (Bangsow et al., 2008). Furthermore, a fraction of the Itm2a IR was detected in the processes of GFAP-positive cells (Figure 3F–H).
Discussion
The pull-down assay showing that Itm2a lacking the BRICHOS domain fails to bind to motopsin suggests that this domain is critical for the interaction with motopsin. The BRICHOS domain is conserved in two other members of the Itm2 family, Itm2b and Itm2c; however, the yeast two-hybrid screen and the co-immunoprecipitation assay failed to detect the interaction between Itm2c and motopsin (data not shown), which suggests that motopsin specifically binds to the Itm2a member of this family. The BRICHOS domain in Itm2a appeared to anchor motopsin around the cell surface, even though this domain is thought to assist proteolytic processing and exert chaperone-like functions (Sánchez-Pulido et al., 2002).
Itm2a is a marker of chondro-osteogenic differentiation (Deleersnijder et al., 1996) and can be induced during thymocyte selection (Kirchner and Bevan, 1999). Our Western blot and immunohistochemical analyses show that Itm2a is abundantly expressed in endothelial cells during the first 2 weeks of life. Vessel sprouting and complex branching occur during this period (Ogunshola et al., 2000). Especially until 9 days, most of capillaries are covered by astrocyte end-feet (Caley and Maxwell, 1970). It is intriguing that Itm2a protein is located on both astrocytes and endothelial cell at this developmental stage. Itm2a may be involved in the development of endothelial cells in the brain. Postnatal development of the vasculature appears to require neuron–endothelium interactions, as pyramidal neurons predominantly express vascular endothelial growth factor at early stages of postnatal development (Ogunshola et al., 2000). Similarly, motopsin in the cerebral cortex is expressed most abundantly during the first postnatal week, and gradually its expression decreases, but continues into adult life (Iijima et al., 1997; Mitsui et al., 2007). Ten days in a mouse life corresponds to several years in humans, during which synaptic density is overproduced in cortical neurons (Kolb et al., 2012). These reports suggest that motopsin also contributes to postnatal vascular formation via the interaction with Itm2a at the epithelial surface, although co-localization of motopsin and Itm2a proteins is undetectable in the developing mouse brain (data not shown), probably because of the low amount of secreted motopsin or the temporal interaction. Furthermore, skeletal muscle cells also express Itm2a (Kirchner and Bevan, 1999). Itm2a is also expressed at sites of myogenesis in the mouse embryo (Lagha et al., 2013). Overexpression of Itm2a enhances the myogenic differentiation of C2C12 cells (Van den Plas and Merregaert, 2004). Overexpression of motopsin in motor neurons in the ventral horn destabilizes the neuromuscular junctions (Bütikofer et al., 2011). Motor neurons in the brain stem and spinal cord express motopsin abundantly throughout the lifespan of an individual, whereas the highest expression of motopsin in the cerebral cortex is observed around the perinatal period. Motopsin may modulate myogenic differentiation or maintenance by interacting with Itm2a at the myogenic cell surface. We have just shown that motopsin modulates neuronal morphology through the interaction with a transmembrane protein, sez-6, on neuronal cells (Mitsui et al., 2013). Secreted motopsin may play multiple functions by interacting to a corresponding binding partner on various kinds of cells. Further investigation should be necessary to understand biological significance of the interaction between motopsin and Itm2a.
Acknowledgments and funding
The anti-myosin regulatory light chain antibody was kindly donated by Dr. Jane E. Ishmael from the Oregon State University. The D-HA vector deposited by Dr. Takemoto was provided by the DNA bank of the RIKEN Bioresource Center, with the support of the National Bio-Resources Project of the Ministry of Education, Culture, Sports, Science and Technology of Japan. This work was supported in part by KAKENHI grants (20591224 and 18591156).