Sequestration of Contaminants from Wastewater: A Review of Adsorption Processes
Abstract
Adsorption has widespread applications in separation processes, particularly aiming at environmental decontamination owing to its economic and highly efficient outcomes. This literature studies various isotherm models concerning adsorption which is accompanied by information related to the mechanism of the adsorption process, which is a crucial aspect of the system design. Many modification techniques bring about a significant enhancement in the working capacity. The build-up of industrial activities causes a spike in the dispersion of toxic compounds along with non-biodegradable contaminants from industrial wastewater. The separation techniques have emerged with greater efficiency in eliminating specific pollutants in terms of discharge legislation. Such methods have been reviewed hereby in this document based on literature published lately. Concerning the process of adsorption, this review elucidates the adsorption isotherms, kinetic models, breakthrough curve modelling, thermodynamic concepts, and the regeneration of the support required in batch or continuous reactors.
1 Introduction
Human beings are confronted with two primary challenges worldwide, namely scarcity of clean water and its pollution. The groundwater and surface water getting contaminated is the root cause of this paucity, whereas the basic sources remain to be natural and anthropogenic 1. The sustainability of life on Earth requires water as a fundamental requirement. The urban activities spanning industries, the agricultural sector, the health domains, and so on, produce a considerable quantity of micropollutants, thereby affecting the aqueous life 2. The domains that have caught the attention of researchers in recent times are environmental pollution and the extensive use of pharmaceutical compounds 3. Compounds that affect the initial water quality ranging from bacteria to industrial discharges result in the formation of wastewater upon its encounter with water bodies. Various reports suggest that a range of pollutants are present in the wastewater from refinery like heavy metals, hydrocarbons, and phenol which induces serious damage to the human body when released untreated 4. The organic pollutants being considered are dyes, volatile organic compounds, and pesticides whereas metals and disinfectant by-products (DBP) such as bromide come within inorganic toxins 5. Worldwide, dyes are produced annually in thousands of tons. Specifically, the annual industrial production of dye corresponds to ∼7 × 105 tons 6. Eliminating these harmful substances has been in the limelight as the chemical industries generate massive amounts of wastewater regularly, which usually finds its way into the open surroundings and gives birth to undesired influences on human vitality and aquatic habitats due to its multi colours and severe toxicity level 7. All the biological, chemical, and physical treatment techniques are implemented to facilitate the elimination of heavy metals as well as dyes found in the wastewater. Out of the myriad of decontamination methods, adsorption stands out because of the availability of various adsorbents, simplicity of preparation, easy handling, minimal operational expense, high efficiency, fast kinetics, extensive applicability, non-secondary contamination, and easy regeneration capacity of the adsorbents 8. As the extent of adsorption is measured by suitable adsorbents for specific components, it is necessary to completely interpret the physicochemical aspects of adsorbents and the mechanisms associated 9. The textural properties and the location of active sites influence the adsorption mechanism 10. The method of adsorption is based on the settling of the impurity over the surface of the adsorbent or among its voids. As the adsorbent plays a crucial role throughout this mechanism, various substances have been studied as they have a probable role as efficient adsorbents. The most regarded among these adsorbents happens to be the application of activated carbon (AC). The property that makes AC the desired adsorbent in the treatment of wastewater is the extensive surface area, leading to remarkable adsorption abilities covering a wide spectrum of toxins. On the other hand, its high cost imposes a hurdle in its extensive use and urged numerous other materials to be studied as an alternate source of adsorbents. This write-up has methodically reviewed the kinds of adsorbents and adsorbates, simulated methods, and adsorption mechanisms and isotherms.
This review gives a basis of adsorption and highlights a theoretical description of the phenomenon under contemplation. The paper deals with common industrial contaminants critically showing the possible adsorbent that can be used for the development of adsorption and presenting some of the state-of-the-art literature on it. Owing to today's application of adsorption science in industry, and in environmental management. The relationship between adsorbent and contaminant and the adsorption theory is focussed. Current perspectives pertaining to challenges are discussed and illustrated.
2 Adsorption Process
Adsorption is a separation process by which adsorbate is attached to the adsorbent surface in an aqueous medium by any form of attractive force (hydrogen bonding, van Der Waals, or hydrophobic bonding) or chemisorption 11. The adsorption process is regarded as a favoured process over others, to seize the organic components out of the water bodies. Regardless, the physicochemical properties of adsorbents would impact the applicability and also the attainment of high values of desired parameters during the process 12. When considered with a low-cost adsorbent, the simple operating system can make its implementation attractive for numerous systems 13. Adsorption can be performed with a lot of adsorbents and comprises a covalent bond developing among the adsorbate and adsorbent that is reversible to a considerable extent 14.
A schematic model of the adsorption process indicating the three associated components and their interactions have been depicted in Fig. 1. The attraction in the middle of the adsorbent and the adsorbate acts as the driving force in the process of adsorption generally in a ternary system. Simultaneously, the affinities existing among the adsorbate and the solution, the adsorbent and the solution, and the toxic components also have a significant contribution to adsorption 15. Adsorption deals with the separation of components present at the juncture of two distinct phases which could be liquid-liquid, gas-liquid, gas-solid, and liquid-solid. The two classifications of adsorption happen to be physisorption and chemisorption, which holds upon the basis of the nature of bonds between the adsorbate and the adsorbent. The adsorbate is the one which remains in contact with the surface utilizing physical forces during physisorption and through the different properties of bonds holding the adsorbate and adsorbent together in case of chemisorption 16. Among these, the former is recognized to be reversible, meanwhile the latter remains irreversible owing to its chemical bonds. The aspect of recycling or regeneration of the adsorbent materials is a major benefit which brings about economic viability 17.
Depending on the mode of the operation, the process is further categorized as static adsorption and dynamic adsorption. Even known as batch adsorption, static adsorption takes place inside a closed system having the exact quantity of adsorbent being contacted with a fixed quantity of adsorbate solution, whereas dynamic adsorption happens under open-system conditions where adsorbate solution incessantly flows past a column filled with the adsorbent 18.
3 Different Contaminants of Concern
3.1 Heavy Metal Contamination
The effluents from industry consist of different organic and inorganic pollutants, which include heavy metals that could probably be toxic and/or carcinogenic thus proving dangerous to humans and other living beings. The ones of major concern here are Pb, Zn, Cu, As, Cd, Cr, Ni, and Hg 19. The risks imposed by the heavy metals being exposed to nature have a connection with toxicity as well as bioaccumulation in living beings or even the food chain given their biological non-degradable property. The toxicity of these heavy metals endangers life forms and thus compels their removal to prevent damage to the environment 20. The variety of paints implemented in the painting process in the graphics domain brings along remains of heavy metals and the cleansing of printing devices dissolves the majority of these components and passes them with the effluents. The graphic industry is commonly found in urban areas and the sewage network is the most probable destination of its effluents degrading the quality of river water flowing through the cities 21.
The issues arising out of pollution caused by heavy metals can be brought down by implementing strategies like precipitation, ultra-filtration, or electro-deposition alongside different techniques, but the drawback of high cost and ineffectiveness in low concentrations exists. Here, adsorption is regarded as an effectual, cheap and environment considerate technique having great scope for discarding, recovery and recycling of heavy metals 22, 23 upon the combination with appropriate desorption stages acts as a solution to the issue of sludge disposal 24.
3.2 Surfactant Contamination
The compound which finds its use in a myriad of industrial and domestic applications is a surfactant. Because of their extensive use in detergent formulation, they are now seen as one of the most general types of contaminants in wastewater. Surfactants are divided into four groups depending on their surface charge, anionic, cationic, non-ionic and amphoteric (zwitterionic). Both anionic and non-ionic surfactants deliver harmful effects due to acute toxicity. However, the non-ionic ones display higher toxicity as compared to their anionic counterparts 25. The use of surfactants has been a usual practice and has seen a rise from the 1980s, emerging from 1.7 million tons in 1984 and 9.3 million tons in 1995 166 to a massive 15.93 million tons in 2014 and a probable figure of 24.19 to be used in 2022 26. The worldwide production of surfactants is above 10 million tons per annum and many daily-use products contain surfactant formulations 27. Membrane treatment and other physical, chemical, and biological techniques are deployed for the separation of surfactants in wastewater. Choosing the best treatment method for this process depends on numerous factors like influent and effluent quality, the environmental footprint, treatment expenses and energy usage 28.
3.3 Pharmaceuticals – Contamination
Pharmaceutical industries are working on the manufacture of healthcare commodities, vitamins, vaccines, hormones, growth parameters, proteins, antibiotics, cosmetic products, cell cultures and so on. Similar to other industries, the waste generated by them is discharged as effluents (liquid waste), termed ‘pharmaceutical effluent', which is a mixture of pharmaceutically active compounds in low concentration, by-products, heavy metals, chemicals, micro-organisms, and cell cultures associated to this process. The threat caused to the environment by more and more of its use and input to the aquatic environment is becoming severe 29. The lack of proper knowledge about the actual consequences on both environment and human health is an added reason for the threat caused by the presence of these emerging compounds in aquatic sources 30.
3.4 Nuclear Industry – Contamination
Among all the longest-lived radio nuclides, uranium (U(VI)) is known to impose serious health issues on humans, specifically at comparatively higher concentrations due to its toxic and radioactive properties. The activities taking place in the nuclear industry, copper mining industry, etc. cause uranium to enter the water streams. Cancer-causing effects of uranium have been well established, and its uptake can result in health damages, particularly in the liver and kidney and eventually leading to death. According to the standards set by the World Health Organization, the permissible limit of U(VI) strength in water is 0.05 mg L−1. Such strict rules have initiated the upcoming of several methodologies for its separation from wastewater arising out of nuclear industries and mining processes 31.
4 Parameters Influencing Adsorption
Several factors including surface morphology, thickness, the porosity of adsorbent alongside adsorbate charge, diameter and their contact duration drastically influence adsorption 32. Some of the factors are depicted in Fig. 2.
4.1 pH
A solution's pH is a prime factor in adsorption as it triggers electrostatic attractions or repulsions amongst the dye molecule and dendrimer-related adsorbent, based on the nature of the dye (cationic/anionic) and/or the solution pH 33. The pH has a direct impact on the metal solubility such that a rise in pH registers a dip in the solubility because of the generation of less-soluble metal hydroxides and oxides arising out of the chelating activity of the ions on the cations, thereby turning the metal soluble in solution which is required for its adsorption. Also, extremely low pH figures indicate a higher concentration of H+ free ions in the solution, in the ion exchange action of the zeolite, improving the clash among the H ions and the metal getting adsorbed, causing their damage (Wang and Peng 70). The adsorption of anionic dyes rises with dropping pH values and behaves vice-versa in the case of cationic dyes 34, 35.
4.2 Adsorbent Dosage
The ideal adsorbent dosage affects the quantity of adsorbed adsorbate and is thus a key parameter. The surface area keeps on increasing with the escalating adsorbent dosage, and knowing an optimal dosage is essential to avoid excess consumption 36. Various research teams have studied the influence of adsorbent dosage on dye or metal removal by adjusting the adsorbent dosage concentration 37-39. The MB removal percentage is dependent on adsorbent dosage in the case of the three studied materials (BMB, Biochar, and CO2 biochar). The trend depicting rising adsorbent dosage brought about a rise in percentage separation of MB which is credited to the large aggregate of adsorption sites 40. The adsorbent dosage upon its increase also increases the percentage of dye removal alongside. Towards the beginning, this increase was seen to be rapid which decelerated as the dose was extracted. The explanation for this phenomenon is that the adsorbate is more conveniently accessible at a smaller adsorbent dose and hence the removal for every unit weight of adsorbent is larger too. However, with an increase in the adsorbent dose, there is a reduced corresponding uprush of adsorption, arising out of the various sites staying unsaturated through the adsorption. This could be due to the fact that there exists rapid superficial adsorption over the adsorbent surface at a higher adsorption amount, and this produces a lower solute concentration as compared to when the adsorbent dose is the lowest 41.
4.3 Particle Size
The adsorption ability steps up with a decreasing adsorbent particle dimension due to the expanding surface area. Although, a scenario where adsorbate is unable to penetrate some of the interior pores may arise, mostly when their dimensions are huge. The smaller-sized particle has easier access to all the pores 42. The influence of the adsorbent particle dimension over the adsorption kinetics had remarkable significance. The rate constant (k) associated with the adsorption by the adsorbent showed a dropping trend with rising particle dimension, highlighting the impact of specific surface area over the rate of adsorption 43.
4.4 Contact Time
In the initial stage, adsorption occurs at a rapid rate and their capacities witness a quick rise at the beginning of the experiment as a result of a large amount of unoccupied adsorption sites on the adsorbent surface. With the duration of adsorption, the sites which were available turn saturated ultimately reaching an equilibrium state 44.
4.5 Temperature
A rise in the temperature leads to a rise in the removal strength, which supports the point that the elimination methods were impulsive, attainable, and endothermic in nature, and can be further stated by the active sites of adsorbent available in addition to the expansion of the pores and surface area at elevated temperatures 45. With respect to the thermodynamic properties and their temperature-related behavior, suggests that the adsorption reaction is enhanced by decreasing temperature, while the desorption reaction can be favored by increasing the temperature 46.
5 Adsorbents
The adsorbent is considered very crucial and is the base of the adsorption process. The ones used in treating water should not just have good adsorption efficiency, cost-effectiveness and eco-friendly properties, but even the properties of simple separation and reproducibility. Currently, different adsorbents are coming with their unique properties, which allow them to be used in their actual structure or a modified version to separate numerous organic or inorganic contaminants from wastewater 47. The main benefit of these cheaper alternatives is that no regeneration is required because of their excessive availability 48. The broad classification of the adsorbents is given in Fig. 3.
5.1 Nano Adsorbents
Nanotechnology presents novel approaches for the water treatment processes in practice by taking some control over the size, shape, and functionality of the materials 50. There are adsorbents-based n nanoparticles (NPs), which possess a huge, fixed surface area, which brings about an adsorption rate noticeably greater than the earlier ones. Nanomaterials owing to their exclusive properties like free surface energy, nano-size the surface volume ratio, catalytic, magnetic, and optical properties have gained the attention of the scientific community for the removal of toxic pollutants 51. These also have an excellent use in cleansing processes, along with being more active and quicker in eliminating inorganic pollutants like denser metals and organic contaminants. Adding to the expense of adsorption devices, the increased efficiency of this technique turns it feasible to execute other complicated methodologies for wastewater treatment. The ones that are studied more commonly are:
Nano adsorbents related to carbon (carbon nanotubes (CNTs)), nano adsorbents depending on metals, polymeric nano adsorbents, and Zeolites 167. In recent years, to decrease the cost of synthesis, wastes with silicon and aluminum have been used for zeolite synthesis, which has developed as a research hotspot 52.
Nanomaterials can be remade without compromising much (6–10 %) on their initial performance. Also, the selectivity of nanomaterials for a particular contaminant among the co-existing and competing ions serves as a direction to explore 53.
The nature of carbon has drawn the attention of researchers in recent times as the morphological, physical and chemical properties associated with it are good to consider. Alongside, adjusting the surface of carbon spheres or introducing a coating of nanoparticles over it would improve its functionality and agreement, which finally ends with applications in several fields 54. Graphene oxide (GO) is considered a possible 2D component having a huge area. Examples like hydroxyl (-OH), carboxylic acid (-COOH), and epoxide groups indicate vast variable functionalities, which are crucial in imparting various advantages to the GO surface for widespread uses. Although adsorption happens easily on the GO surface, the extension to practical implementations has not been possible due to the severe deposition of GO in the solution in terms of its excessive hydrophilicity. Various surface manipulations have been tested to allow recycling of GO and enhance adsorption ability along with its potential to deal with this drawback. Magnetic graphene oxide is a composite component made of graphene oxide along with a magnetic component like iron oxide. The nano adsorbents based on phosphorene-oxide are being suggested for the concurrent elimination of very harmful inorganic species like As(III) and As(V) from water. They display some typical benefits like: i) adsorption mechanism through surface complexation of the inner portion of the sphere, ii) good adsorption efficiency in addition to moderate oxidation degree, and iii) large recovery owing to easy treatment techniques using alkaline eluents. Hence, these become highly probable options for future control and corrective methodologies for arsenic pollution in water 46.
5.2 Carbon-based Adsorbents
The materials out of carbon are either finely crushed or rather large non-metallic solid ones having a major portion filled by carbon including activated carbon/biochar (AC/BC), carbon nanotubes (CNTs) and graphene (GN) analogues, etc. Additionally, they are known for the added benefit of greater area, ample pore layout, commendable thermal stability, great mechanical strength, great adsorption ability and manageable morphology 56.
5.3 Activated Carbon
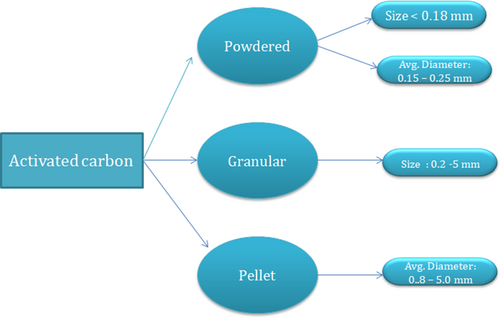
The earliest recognized adsorbents are the activated carbons (ACs). They come with a highly porous configuration having a huge inner surface area varying from 500 to 2000 m2g−1 and as an impact has good adsorption capacities for several substances. It has found its use in separating a range of pollutants containing organic and inorganic components from the liquid or gaseous state. They are found in several forms as displayed in Fig. 4: powdered activated carbon (PAC), and specifically, granular activated carbon (GAC). Among them, PAC consists of fine particles, smaller than 0.2 mm in diameter, and hence exhibits large external surface area and small diffusional resistance. This results in a high rate of adsorption. On the other hand, GAC is made of larger carbon particles measuring up to 5 mm in diameter, accompanied by smaller external surface areas in comparison with PAC.
The activated carbon gets attracted by organic compounds and has oxygenated functional groups present over the complete surface, which attracts the attention of organic pollutants 58. Different residues like industrial activity ash, rice husks, coconuts, residual sludge, peat, with more, are being put to use for the making of activated carbon 59. Activated carbon has a lot of characteristics to offer including a definite surface area, high porosity and pore volume, and changeable surface characteristics with respect to morphology and functional groups. Its applications have been widespread, inclusive of water treatment. It is procured by the thermochemical transformation of organic materials at elevated temperatures and void of oxygen. The factors like the raw material, production methodology, and constraints have all portrayed a vital part in enhancing its adsorption efficiency 60.
The activated charcoal usually adsorbs the inhibitors much more effectively than the polymer resin adsorbents, and it is further complicated as well as expensive to recover them from activated charcoals than from polymer resin adsorbents. As activated charcoals are not apt for recovering the inhibitors, the separation of the inhibitors from lignocellulosic biomass hydrolysates with polymeric resin adsorbents is being considered 61. AC for commercial purposes is generally obtained from carbon-based materials like coal, peat, petroleum coke, etc. The major disadvantage associated is their costly reactivation processes as well as greater regeneration energies that occasionally cause decay in the case of solid adsorbents post several cycles.
5.4 Polymers
The natural and synthetic types of polymers find an application as adsorbents. The polymeric adsorbents bring along their advantages and limitations too. Their main advantage lies in their easy modification to attain selectivity/affinity for certain pollutant types based on different chemical alteration methods. While the prominent drawback remains its price as it is relatively expensive to synthesize as compared to other adsorbent classes 62. Synthetic polymers are seen extensively in the retrieval of natural polyphenols because of their selectivity, efficiency, regenerative ability, minimal cost, chemical inertia, and minimal toxicity 63. Cellulose acts as a biodegradable, non-meltable and renewable polymer that is also insoluble in a majority of the solvents because of its hydrogen bonds and crystalline properties. Natural cellulose has smaller adsorption strength unlike modified cellulose, and this can be improved by manipulation using chemicals. This can be used as an adsorbent, but cellulose is also modified due to its low adsorption capacity of the former one. Cellulose itself and many of its products do not affect the environment as they come back to the natural carbon cycle through a simple decay with decomposers. They also have a typical physicochemical property of having great sorption power, thus turning them into a perfect adsorbent in their natural and modified forms 169.
Cyclodextrin polymers function as effectual adsorbents for the rapid adsorption of heavy metal ions from the environment 64. The coordination polymers (CPs) are being considered more for its empty channel, composition distinction optimum structural diversity and different dimensions and configurations. This makes it suitable to be used for catalysis, gas storage and delivery. Specifically, in comparison, CP is advantageous due to its easier attainment of functionalization and diverse preparation methods along with being the best adsorbent for separating metal ions. The low chemical stability, low adsorption ability and meagre selectivity of many CPs become a hurdle in their application in aqueous solutions. This makes the method of improvisation of material performance key to its application 65. Being a polysaccharide derived from sesbania seeds, sesbania gum (SG) is organic, chemically non-reactive, non-toxic, cost-effective and seamlessly available. Considerably, the plentiful hydroxyl groups present on the SG surface' provides versatile probabilities for covalent functionalization. It is being widely studied as a side substrate in numerous cases including adsorption, catalysis, flocculation, textiles, pigments, and cosmetics. Specifically, SG has been identified to be a suitable adsorbent for combating environmental pollution because of the ample surface functional groups and excessive adsorption efficiency 66. In recent times, hyper cross-linked polymers (HCPs) are known to be effective organic polymer-based adsorbents which can be used for wastewater treatment. Due to the hydrophobic characteristics of HCPs arising out of rich aromatic systems in their skeletons, they can very well eliminate oleophilic-hydrophobic pollutants with its base being hydrophobic interaction, van der Waals interaction and π–π interaction amidst the target pollutant molecules and adsorbents. Although, when HCPs are subjected to hydrophilic or water-soluble toxins like metal iron, water-soluble dye and antibiotics, the adsorption capability is significantly diminished 67.
5.5 Clay
Clay has a final negative charge giving it vital cation adsorption and cation exchange characteristics. Its arrangement and the aqueous part's pH decide the onset of adsorption and other exchange characteristics of the clay. Clay being implemented in environmental applications is known for the adsorption of dangerous contaminants like transition metals, and organic micro-pollutants (example: biocides, dyes) in wastewater. Still, the affinity of clay towards phosphate adsorption is quite low. Allophane is an exception as it has various environmental uses owing to its reaction seen with harmful anions. The reasons behind the high retention capacity for anions are the small particle size, great area occupied on the surface and the aluminol groups found on the surface 168. Over decades, the adsorbent employed to eliminate heavy metals, and dyes in aqueous solutions are natural and synthetic clays. Over the past few years, shedding natural clays into low-dimensional nanosheets or nanotubes for making multifunctional clay-based adsorbents is gaining a lot of importance. Because of the complicated porous structure having a large specific surface area, the internal, as well as external surfaces of clays, get to deal with dissolved species. In the case of many clay minerals with negative surface charges, the factor causing this is the isomorphic substitution in the tetrahedral sheets (Al3+ for Si4+) and/or in the octahedral sheets (Mg2+ for Al3+), and cationic contaminants will be attracted. The ones that occur naturally like Ca2+, Mg2+, and Na+ are found in between or over the surface to adjust the charge, making the clay instilled with an ion-exchange capacity to manage the pollutant 68.
With respect to the adsorption properties of the clay mineral, surface modifications have been considered as depicted in Fig. 5 as they permit the coalescence of new materials and as a result, the introduction of new areas of usage. Their preparation can be made possible with sol-gel synthesis at room temperature, co-precipitation, and microwave-aided blend with numerous molecules. The functional groups which are found on interlayer clay mineral surfaces play an important role, and the mentioned organic functional groups are enabled to provoke certain interactivities with adsorbate molecules. Various environmental, biological, and catalytic activities need the synthesis of functional clays to be greatly dissolvable in an aqueous medium and capable to bond with required substrates (adsorbates) 22.

5.6 Industrial Waste
Some of the wastes generated by the industries are being used in the form of adsorbents for the adsorption of ions and organics in wastewater 69. This use of waste is becoming popular because of its easy availability, minimal cost, and good mechanical and chemical resistance. This application is in line with the concept of green chemistry and also with the booming environmental regulations enforced to prevent improper disposal practices 70. The reuse and recycling of industrial garbage have been explained through the circular economy, sustainable development, along with green engineering techniques. The fate, transportation, and transfer of disposed of graphite impurity in soil, water and air components because i) environmental problems acting as the absorber and carrier of harmful compounds, ii) acute health issues like graphitizes because of unwanted graphite exposure, and iii) social disturbance as the dense graphite dust obstructs visibility 71. Mechanically activated fly ashes can be employed as an adsorbent for separating a few heavy metals namely, Cu(II), Mn(II), Ni(II), Pb(II), and Zn(II) from aqueous solutions. The adsorption ability of fly ash can be varied by enhancing the surface activity with mechanical activation 72.
5.7 Natural Adsorbents
The natural biomasses being used as adsorbent is grabbing more and more attention because of their biodegradability, biocompatibility, and renewability 73.
Biosorbents are the category of adsorbents obtained from biomass and related materials which do not take part in any thermochemical breakdown prior to being used. The important aspect of biosorbents is their retention ability towards the actual composition of the source material. In terms of biomass, the degrading of components starts at a temperature greater than 300 °C. Although, material processed below this temperature is also considered a biosorbent. Further, even without having an extremely large surface area, the numerous functional groups provide it with the ability of dye uptake through various physicochemical interaction mechanisms 62.
Biosorption implements a few biological materials such as chitosan, yeasts, fungi, chitin, and bacteria in order to separate toxins in the mixture using chelation and complexation. Considered alongside the conventional ion exchange resins and commercial AC, the biosorption materials show an improved selectivity in addition to the toxin quantity brought down to the ppb range. Biosorption is an ideal technique to eliminate contaminants at a very minimal price. It also has an added advantage of a high elimination rate specifically for extremely toxic wastewater. Further, an easier path of fermentation technology and a cost-effective culture substrate can be used because of the lesser production expenses of fungal biomass. Also, biosorption is an expanding method which managed to surpass the imperfections of selectivity in conventional adsorption processes. Meanwhile, bio-adsorption is also known to have some unfavorable factors associated with it. The first of them is that adsorption is a slow process, followed by the other is that the pH has an effect on this process. Also, the functional groups and surface features in fungal organisms show an effect on adsorption. Moreover, some additional factors also altered the adsorption process, namely competitive salt and ion concentration. In addition to this, the biomasses could restrict the adsorption column easily 74. Among biosorbents, Chitosan has some disadvantages in terms of being soluble in low pH as it does not adsorb Cr (total) at small pH levels. This happens due to reason that the active site (amine group) in Chitosan goes through protonation and its adsorption ability gets affected by anions in water 75. Tab. 1 lists the Adsorption efficiencies of various adsorbents.
|
Efficiency [%] |
Adsorbate |
Adsorbent |
Ref. |
|---|---|---|---|
|
99.95 |
Methylene blue (MB) and cationic yellow (CY) |
Magnetic silicate@Fe3O4 |
68 |
|
70 |
Yellow dye |
Natural clay |
76 |
|
100 |
Carbendazim |
Bentonite clay |
77 |
|
92 |
Cd |
Natural clay |
78 |
|
100 and 93 |
MB dye and methylene orange |
Industrial carpet waste |
79 |
|
99.17 |
Azodye |
Industrial waste |
80 |
|
90 |
Cationic dye |
Ceramic adsorbents prepared from industrial waste |
81 |
|
82.7 |
As |
Slag material |
82 |
|
88.7 |
Industrial toxic dye |
Moringa seeds |
83 |
|
97 |
mixed dyes |
Industrial microbial waste |
84 |
|
97 |
MB |
Rice straw |
85 |
|
96.6 |
Heavy metals |
Organic-inorganic hybrid of poly(acrylic acid-acrylonitrile)/attapulgite, P(A-N)/AT nanocomposites |
86 |
|
94.85 and 92.78 |
Pb |
Mango seeds cover with kernel, and jamun seeds cover with kernel |
87 |
|
99 |
Cr, Cu, Pb, and Hg |
Activated carbon and biochar, and nanomaterials |
88 |
|
86.7 |
Cd(II) ions |
Chitosan composite |
89 |
|
98.71 |
U(VI) |
Brown algae |
90 |
|
85 |
Heavy metal |
Desiccated coconut |
91 |
6 Qualities of Good Adsorbents
The salient characteristics of an adsorbent in any application are its capacity, selectivity, reconstructibility, kinetics, compatibility, and expense 92. Along with this, an excellent adsorbent must also possess the following characteristics. Fig. 6 illustrates the qualities of good adsorbents: (1) good reusability, (2) distinct pore structure to enable rapid interaction between adsorbate and adsorbent, (3) high thermal stability, (4) high surface area 93, (5) reversibility of adsorption, and (6) easy renewability 94.
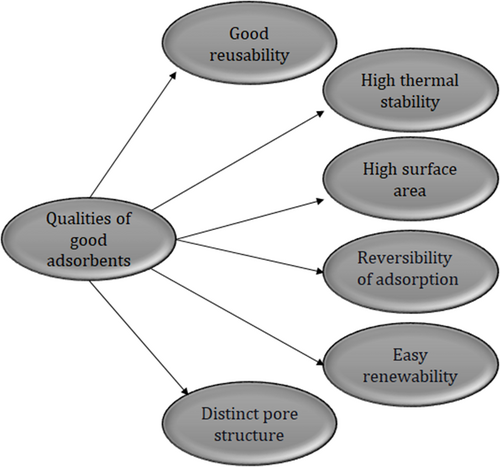
Appropriate functional groups have to be chosen as adsorption spots for strong adsorption forces like primary, secondary and tertiary amines for interactions among the ions. Alongside, a certain amount of overall adsorption sites and decent adsorbent accessibility is necessary for the dyes. Thus, an adsorbent must have a plausible amount of adsorption sites for every unit of surface area 95. The adsorbents are generally in granular form having dimensions from 0.5 mm to 12 mm, and they should not provide a high-pressure drop or even get taken away with a stream flow. Their shape and size should remain intact amidst handling, and they should have a larger surface area per unit mass along with too many pores 96.
7 Mechanism of Adsorption
Based on the factors governing the activities at the solid surface and the adsorbate, adsorption is categorized into chemisorption and physisorption. Among these, chemisorption takes into account the build-up of a chemical bond, either ionic or covalent, between the adsorbent and the adsorbate, accompanied by an enormous exothermic enthalpy and induces a modification in the electronic configuration of the adsorbate as well as the adsorbent. Whereas physisorption has weaker forces involved like Van der Waals and polar interactions, being associated with a relatively smaller enthalpy causes some agitation in the electronic structure of the compounds associated 97. Considering physisorption, the drop sticks onto the surface because of Van der Waals's force of attraction, surface roughness or hydrophobicity. This accumulation takes place over the surface in a mono-layer or multi-layer form based on process characteristics. In the case of the monolayer build-up, the solute builds up in the form of a single layer with one molecule depth, and in the multilayer one, the solute accumulates in multiple layers comprising changing chemical bonds and physical forces in several layers. And the multilayer accumulation will give higher adsorption quantities, but owing to the number of bonds associated, the pores are required to be sufficiently big to adjust the bond lengths 98.
8 Adsorption Isotherm
The mechanism concerning the adsorption of natural and synthetic adsorbents has been revisited in terms of thermodynamic, equilibrium, and kinetic studies. The isotherm models explaining the adsorption equilibrium were studied on the basis of several isotherm models. Depending on the assumptions that these patterns are derived, it could be estimated whether the adsorption processes turn out to be homogeneous or heterogeneous, or if an amalgam of both took place on the surface. Additionally, they also suggest if the process happened favorably, unfavorably, chemically, or physically 99. The isotherms are vital in explaining the interactions among adsorbent and adsorbate that are useful in understanding the process 100.
Adsorption isotherms are affected by: adsorbent properties, adsorption cycles and desorption differ adsorbent properties likely because of the gradually developing changes in the pore structure, origin and preparation technique, in addition to temperature and relative humidity of the gaseous stream 170.
Along with the ongoing adsorption process, the desorption and adsorption rate will acquire a similar stage with time, which indicates that the kinetic model and adsorption isotherm are usually made to adjust with the adsorption 101. Adsorption isotherms are derived from conducting batch studies at constant temperature values. This constant figure estimated through the isotherm equation gives an equilibrium adsorption capacity plus an affinity for the adsorbents.
Isotherms describing adsorption of organic compounds are spread over four major groups based on the behavior of slope at the starting part of the curve, and further into sub-categories.
The main categories are: (i) S curves, indicating vertical inclination of molecules getting adsorbed, (ii) L curves, the normal or Langmuir isotherms, mostly suggesting the molecules adsorbed on the surface, or, occasionally, of vertically arranged adsorbed ions with specifically severe intermolecular attraction, (iii) H curves (high affinity), usually brought by solutes adsorbed in the form of ionic micelles, and through high-affinity ions exchanging with low-affinity ions, and (iv) C curves (constant partition) and linear curves, coming from solutes which pass through into the solid much easily than the solvent. The sub-groups of these categories are considered based on the configuration of the sections of the curves that are away from the origin. Therefore, if the solute molecules getting adsorbed are set in a way that the novel surface has very less attraction for further solute particles and the curve thus has a long plateau; and if adjusted such that the new surface has a greater attraction for the further solute, the curve sees a steady rise without any plateau 102.
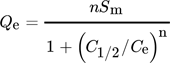 ()
()There are three parameters inculcated into this model, where n stands for the number of adsorbate molecules for every adsorbent site, Sm denotes the density of receptor sites and C1/2 represents the concentration at half-saturation. Accordingly, the adsorption capacity at saturation can be determined by Q = nSm.
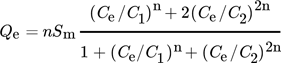 ()
()In this type of adsorption, the dual concentrations at half-saturation are figured and matched to the development of the two initial layers, respectively; they are mentioned as C1 and C2. Accordingly, the adsorption capacity at saturation can be calculated by: Q = 2nSm 103.
8.1 Langmuir isotherm
The Langmuir isotherm is developed on these assumptions: (1) the adsorption process occurs as a monolayer process (chemical adsorption), (2) homogeneous adsorption site which could be adsorption pellets, and (3) an unchanged adsorption heat with the coverage. Thus, as per the Langmuir isotherm, adsorption occurs on the collision of a free adsorbate molecule with an unoccupied adsorption site and also when every adsorbed molecule has a similar desorption percentage 104.
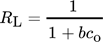 ()
()The value of RL showcases the process feasibility. It holds favorable at 0 < RL < 1 and unfavorable at RL > 1. RL = 0 conveys that the adsorption reaction has attained equilibrium whereas RL < 0 conveys that the adsorption in this case is irreversible 105.
8.2 Freundlich Isotherm
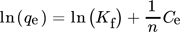 ()
()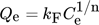 ()
()where Ce is the equilibrium concentration (mg L−1), kF stands for the Freundlich isotherm constant based on the adsorption capacity; n for the adsorption intensity, and Qe is the quantity of adsorbate in the adsorbent at equilibrium (mg g−1) 108. The value of n is a crucial aspect, acting as a sign of the curvature of the isotherm. If the value of n is found to be 1, then the process is said to be linear, and if n is greater than 1, then it is a favorable physical process. Similarly, if n has a value below 1, the adsorption is termed as a chemical process and is unfavorable 109. An adsorption process is termed co-operative based on the 1/n values, wherein a value of 1/n greater than 1 suggests a high co-operative process and its counterpart stands for a less co-operative process. Additionally, a decreasing value of 1/n leads to a heterogeneous process whereas the process becomes more efficient at low adsorbate concentrations 110.
8.3 Sips Isotherm (Langmuir-Freundlich) Equation
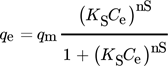 ()
()Here, KS is an affinity constant and nS indicates surface heterogeneity. If nS is equal to 1, Sips isotherms tend toward the Langmuir isotherm and suggest homogeneous adsorption. Also, the deviation of the value of nS from 1 indicates surface heterogeneity. At high concentration levels, Sips reach a constant value whereas Freundlich isotherm is applied in case of low concentrations 112.
8.4 BET (Brunauer-Emmett-Teller) Isotherm
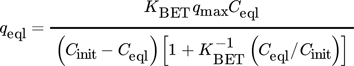 ()
()where Cinit is metal ion concentration at saturation (in mmol L−1) and KBET is a parameter based on the intensity of binding of all the layers; There are two limiting scenarios identified here, when Ceql << Cinit and KBET >> 1, this model tends towards Langmuir isotherm (KL = KBET/Cinit) 114.
8.5 Redlich-Peterson Isotherm
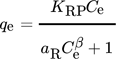 ()
()where KRP and b are the constants. With b getting equal to 1, the model takes the form of Langmuir Isotherm and meanwhile it deduces to Freundlich isotherm, in scenarios where the value of the term aRCβe goes above 1. The ratio of KRP/aR stands for the adsorption capacity 115.
8.6 Temkin Isotherm
Initially, the Temkin empirical isotherm model was implemented for the explanation of the adsorption of hydrogen on platinum electrodes kept in an acidic solution which acts as a chemisorption system. This system takes into account the interaction between the adsorbent and the adsorbate while neglecting the high and low concentration values 116. It also assumes that the adsorption heat varies along with temperature, and that of all the molecules remaining in the layer, it drops linearly and not logarithmically as a result of the expanding surface coverage owing to the sorbent–sorbate interactions 117.
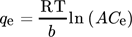 ()
()where A and b are constants 118.
8.7 Dubinin-Radushkevich (D-R model)
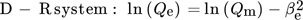 ()
()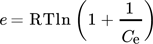 ()
()where β (mol2J−2) represents the adsorption energy constant, e (kJ mol−1) is the potential Polanyi energy obtained from Eq. 10, Polanyi sorption potential denoted by ε is the amount of work needed to shift a molecule from its position in the sorption space to infinity, independent of temperature 121. R (8.314 J (mol K)−1) is the molar gas constant; T (K) is adsorption temperature; Qe (mg g−1) and Qm (mg g−1) are the equilibrium and maximum adsorption capacities respectively, and Ce (mg L−1) is the equilibrium constant 122. Various adsorption isotherm models were applied and the best fits obtained are summarized in Tab. 2.
|
Adsorbent |
Adsorbate |
Models best fit |
Models tested |
Ref. |
|---|---|---|---|---|
|
Date seeds |
Methyl violet dye |
Langmuir |
Langmuir and Freundlich |
123 |
|
Fly ash |
Tannery effluent |
Freundlich |
Freundlich |
124 |
|
Synthetized cobalt oxide |
Phenol |
Langmuir |
Freundlich model and the Langmuir model |
125 |
|
Papaya bark fiber |
Methylene Blue |
Langmuir |
Langmuir and Freundlich adsorption isotherms |
126 |
|
Palm kernel shell |
Methane |
Freundlich |
Langmuir, Freundlich, and Temkin |
127 |
|
Carbon nanotubes |
Metal ions |
Redlich-Peterson |
Redlich-Peterson, Sips, Langmuir, Freundlich |
128 |
|
Kaolin and kaolin/TiO2 nanoadsorbents |
Pollutants from industrial wastewater |
Redlich-Peterson (R-P), |
Langmuir, Freundlich, Halsey, Jovanovic, Redlich-Peterson (R-P), Flory-Huggins |
129 |
|
Cashew nut shell activated carbon |
Brilliant green dye |
Langmuir isotherm |
Langmuir isotherm |
130 |
9 Kinetic Models
The two major aspects to understand the processes of adsorption and desorption are equilibria and kinetics. With respect to these processes, thermodynamics only helps us with data about the ultimate state of a system whereas kinetics is concerned with variations in chemical properties with time and in all the rate of changes 131. The kinetic theories indicate the adsorption rate and are studied in terms of the reaction-based models (pseudo first-order, pseudo second-order) and diffusion-related models. Various kinetic models were tested and the best fits obtained are summarized in Tab. 3.
|
Adsorbent |
Operating parameters |
Models best fit |
Models tested |
Ref. |
|---|---|---|---|---|
|
Activated carbon in the form of carbonaceous hydrochar |
pH, time, and concentration |
Linear pseudo second-order kinetic model |
Pseudo first-order kinetic (PFOK) model and pseudo second-order kinetic (PSOK) model |
132 |
|
Turkish lignite |
Contact time, dye concentration, temperature, pH, adsorbent dosage |
Pseudo second-order kinetic model |
Pseudo first-order and pseudo second-order model, intraparticle diffusion |
133 |
|
CTMAB-Bent |
Adsorbent dosage, solution pH, contact time and dye concentration |
Pseudo second-order |
First-order, pseudo second-order, intra-particle diffusion and Elovich |
134 |
|
Simarouba glauca seed shell carbon |
Adsorbent dosage, contact time, pH |
Pseudo second-order kinetics |
Pseudo second-order kinetics, intra-particle diffusion |
135 |
|
Sugarcane bagasse |
Contact time, adsorbent dose, dye concentration, temperature, pH, and NaCl dose |
Pseudo second-order |
Pseudo first-order (PFO), pseudo second-order (PSO), and intra-particle diffusion (IPD) models |
136 |
|
Tea waste/Fe3O4 magnetic composite |
Adsorbent dosage, dye concentration, contact time, pH |
Pseudo second-order |
Pseudo first-order, pseudo second-order, Elovich, intra-particle diffusion model |
137 |
|
A-zeolite/bacterial cellulose composite membrane |
Dye concentration, adsorbent type, contact time, temperature, and pH, |
Pseudo second-order |
Linear and non-linear forms of pseudo first-order, pseudo second-order, and intra-particle diffusion models. |
138 |
|
Bio-nanocomposite based on whey protein nanofibrils and nano-clay |
Temperature, and pH, adsorbent dose, dye concentrtaion |
Pseudo second-order and intra-particle diffusion |
Pseudo first-order , pseudo second-order, and intra-particle diffusion |
139 |
9.1 Pseudo First-Order Kinetics
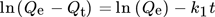 ()
()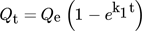 ()
()where Qt is the quantity adsorbed at time t, Qe for the equilibrium amount, t for time (min), and k1 is the rate constant 140.
9.2 Modified Pseudo First-Order Model
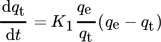 ()
()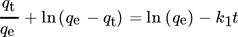 ()
()This is the final equation for the modified pseudo first-order reaction, where K1 denotes the rate constant of the modified pseudo first-order (min − 1) 141.
9.3 Pseudo Second-Order Kinetics
 ()
()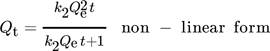 ()
()9.4 Kinetic Model of Intraparticle Diffusion
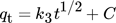 ()
()k3 (mg g−1min−1/2) stands for the equivalent adsorption rate constants, and qe (mg g−1) and qt (mg g−1) stand for the Pb(II) and Ni(II) ions getting adsorbed at equilibrium and at time t, respectively 145.
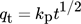 ()
()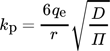 ()
()where, r stands for particle radius and D for intra-particle diffusivity 147.
10 Thermodynamic Considerations
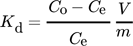 ()
()where Kd denotes the equilibrium distribution coefficient, Co (mmol L−1) for the initial concentration, Ce (mmol L−1) is the equilibrium concentration in solution after being adsorbed and V (L) is the volume of solution. The distribution coefficient (Kd) denotes the ability of clay minerals to hold a solute and the extent of its mobility in a solution phase. Kd holds a significant role in comparing the adsorption capacity of different clays for specific ions under identical experimental conditions. Moreover, Kd values are implemented in the graph of ln (Kd) versus 1/T so as to estimate the Gibbs energy change (ΔG0) of adsorption at various temperature values 148.
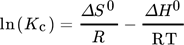 ()
()where Kc denotes the distribution coefficient for the adsorption, ΔH0 is the enthalpy change, ΔS0 for the entropy change, ΔG0 denotes Gibb's free energy change, R stands for the gas constant and T is the absolute temperature 149.
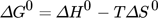 ()
()R is equal to 8.314 J (mol K)−1 and T is the absolute temperature (K). The graphical derivation of thermodynamic features was performed by Haghighizadehet et al. 172. The absolute magnitude of standard free energy change (ΔG0) for physisorption falls in the range of 0 and −20 kJ mol−1, while for chemisorption it ranges from −80 to −400 kJ mol−1 150.
If the standard enthalpy ΔH0 has a value lesser than zero, it suggests that the adsorption process is of exothermic nature, Rahdar et al. 152. The negative value of Gibbs free energy (ΔG0) indicates the spontaneity of the process and the positive enthalpy (ΔH0) figures of RhB adsorption onto MgO-FCM-NPs suggest an endothermic nature.
Thermodynamic parameters supply the required data to develop an adsorption mechanism. Mostly, thermodynamic parameters, namely heat of enthalpy (ΔH), Gibbs free energy (ΔG), and entropy (ΔS) are important features that determine the feasibility and spontaneity. For estimating these parameters, KL (Langmuir constant), Kd (solute coefficient distribution), and Ce (equilibrium fluoride concentration) which depend on temperature are given by Eqs. 24–31.
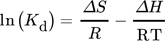 ()
()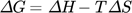 ()
()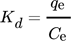 ()
()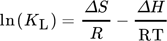 ()
()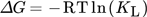 ()
()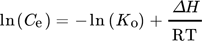 ()
()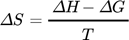 ()
()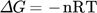 ()
()where n is the Freundlich constant 152.
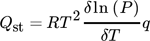 ()
()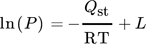 ()
()where L is the integration constant and ΔH = –Qst 153.
11 Regeneration and Reuse
An adsorbent to be effective should have a high adsorption efficiency plus regeneration capacity. The study related to regeneration is important to find out about the recovery of pollutants and adsorbent compatibility 173. Hence, the regeneration of support that has been used in the batch or the dynamic adsorption is a required aspect to minimize the expenses. And therefore, the regeneration and desorption experiments help to determine the ideal desorption of an eluent 154. Tab. 4 represents a summary of the regeneration studies done on certain adsorption studies.
|
Study |
Regents of regeneration |
Regeneration efficiency [%] |
Ref. |
|---|---|---|---|
|
Sb-SnO2/C membrane to treat phenolic wastewater |
NaOH |
80.6 |
155 |
|
Removal of organic micropollutants from wastewater effluent: selective adsorption by a fixed-bed granular zeolite filter |
Ozone |
∼70 |
156 |
|
Removal of ciprofloxacin from wastewater by intercalated Ti3C2Tx MXene |
NaCl |
∼60 |
157 |
|
Coupling ammonia nitrogen adsorption and regeneration unit with a high-load anoxic/aerobic process to achieve rapid and efficient pollutants removal for wastewater treatment |
NaClO–NaCl |
∼94 |
158 |
|
Adsorptive treatment of coking wastewater using raw coal fly ash: adsorption kinetic, thermodynamics and regeneration by Fenton process |
Fenton's reagent |
89.7 |
159 |
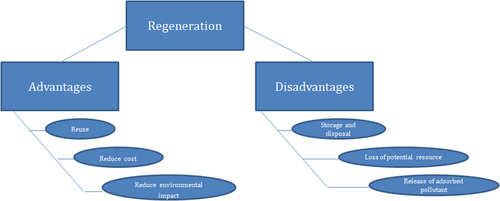
Regeneration is a special technology which has the ability to restore the adsorption ability of used adsorbents by desorption of the impurities already adsorbed. This is generally a cost-effective and better alternative than changing the adsorbents altogether. This process varies with experimental temperature, pH, time, and the cycles necessary for processing 160. Additionally, if the adsorbent is not amenable to regeneration, it has to be discarded after every use, which is one of the main drawbacks of this technique 161. Post the adsorption process, the used dye adsorbents are removed as waste, but a decline in waste generation, regeneration, and reuse of the deployed adsorbent are noteworthy. Out of these, the regeneration process should be economical and with less energy consumption 162. With respect to the environmental, economic and practical aspects of view, regenerability is a key point in estimating the efficiency of an adsorbent. The advantages and disadvantages of the regeneration process have been shown in Fig. 7. The definition of regeneration describes it as the continuous recycling or recovery of the used adsorbents with methods that are viable both technically and economically 163.
The process of desorption is one of the economical and effective regeneration process, reserving the process of adsorption. Research on desorption gives a clear image of the kind of adsorption which can turn out to be either physical or chemical adsorption. It also emphasizes the performance of the adsorbent for further use, causing a reduction in related expenses. Desorption is classified into 1) thermal desorption, where desorption is conducted with heat. Keeping its speed and efficiency aside, this process involves heavy expenses, pre-treatment which liberates toxic contaminants, associated unsafe process conditions, challenges due to regulations and public perceptions, etc. 2) Solvent desorption, which is environmentally feasible, cheap, fast, and has high potential 55, 164. Adsorbent regeneration is a process that can be brought about by numerous methods like temperature swing regeneration (TSR), pressure swing regeneration (PSR), reactive regeneration, or altering the envelope around the adsorbent with a fluid which can extract the adsorbate. Within these mentioned methods, TSR and PSR are widely used for adsorbent regeneration, although, TSR is mostly used for purification purposes 165.
12 Challenges
When novel sorbents are being implemented for wastewater treatment, a major issue that accompanies is an ineffective or slow rate of desorption/regeneration. At present, the boundaries on adsorption keep an extensive use of technological innovations away making it difficult to tackle the dearth of regeneration techniques to facilitate the recycling of the adsorbent. As a result, the pollutants end up being disposed into the environment without the process of regeneration. The compounds acting as regenerative agents are hazardous to the polymer backbone of the biosorbents, and hence finding a better alternative poses a challenge to the researchers. Also, the gaps found demand further research to develop a cost-effective modification technique. The waste-derived adsorbents have to be modified by means of either physical or chemical processes owing to their inherent shortcomings. There has been considerable development in terms of the introduction of new adsorbents, but they are yet inapplicable as they need a comparatively acidic environment and create sludge at the end even though they have been tested and improved over time.
13 Conclusion
From the study, we see that separation of contaminants should be done through unique adsorbents. Properties such as their pore dimension, alkalinity, negative charge, exceptional permeability, and surface area along with their cost-effective and sustainability should be considered. This review discusses the type of contaminants and the different molecules that can be used as adsorbents. Despite the successful use of different materials, there are still various limitations, making commercial and practical use difficult. Therefore, there is a need for further development for enhanced adsorption capacity to adsorb various types of environmental pollutants. From the cost perspective, the ability of adsorbents to be recycled is also an important criterion in industrial applications.
Apart from the adsorbent type, there are various physical and chemical parameters that play an important role in the process. So, theoretical knowledge is necessary to understand the interaction mechanism between the contaminants and adsorbent. The theoretical background and physical meaning for the most relevant adsorption kinetic models was reviewed and discussed. The empirical pseudo second order and pseudo first order models are adaptations to heterogenous systems; however, they can be obtained from fundamental theories such as Langmuir kinetics. For selecting the optimum model , assessment and comparing the error functions such as R2, RMSE (root mean square error), χ2 (chi-square) and so on are essential. Calculating R2 value is a common practice for evaluating fitting results. However, the differences in R2 values are very small in some cases; hence calculating R2 alone is insufficient for model comparison. The model parameters are strongly dependent on the operating conditions. It is recommended to use the complete isotherm to correlate with the experimental data. The adsorption mechanisms and surface properties are not directly related to the isotherm models.
The process of adsorption brings along features like simplicity, high efficiency and extensive availability making it an emerging treatment process for dye removal. This also pushes a need for adsorbents that perform with more efficiency in the domain of water treatment which would enhance pollution control at large scale for commercial applications.
This review paves a foundation for the researchers in chemical engineering by giving an idea about each module involved in the process of adsorption related to exclusion of contaminants from wastewater along with the explanation of the mechanism or kinetics. On the basis of the diverse contaminants and adsorbents discussed here, new researchers can choose the right combination of adsorbent and contaminant for the treatment process.
Conflicts of Interest
The authors declare no conflict of interest.
Authors Contributions
Dr. Divyashree Somashekara has contributed to the write up and Dr. Lavanya Mulky has conceptualized the manuscript content.
Abbreviations
-
- AC
-
activated carbon
-
- BC
-
biochar
-
- CDP
-
cyclodextrin polymers
-
- CNT
-
carbon nanotubes
-
- CP
-
coordination polymers
-
- CY
-
Cationic yellow
-
- GAC
-
granular activated carbon
-
- GN
-
graphene
-
- GO
-
graphene oxide
-
- HCP
-
Hyper cross-linked polymers
-
- MB
-
Methylene blue
-
- PAC
-
powdered activated carbon
-
- SG
-
Sesbania gum
-
- TSR
-
Temperature swing regeneration
Biographies

Divyashree Somashekara is an Assistant Professor Selection Grade in the Department of Biotechnology at Manipal Institute of Technology, Manipal, India. She received an M.Sc. degree in Microbiology from the University of Mysore, Mysore, and her Ph.D. in microbiology from Central Food Technological Research Institute, India. Her current research interests include the production of microbial polymers/bioplastics, the role of microorganisms in the production of value-added products by biotransformation, bioremediation of toxic compounds released in the environment, and variolation of waste and also probing micro RNAs to the metabolic disorders.

Lavanya Mulky is an Assistant Professor at the Manipal Institute of Technology, Manipal Academy of Higher Education, Manipal, Karnataka, India. She also received her doctoral degree from the same university in 2021. Her scientific focus lies in the areas of erosion -corrosion, microbial corrosion in industrial metals, hydrodynamics of biofilms, bioremediation of pollutants, bio adsorbents for oil water emulsions.




