Three New Polyprenylated Benzophenone Derivatives Isolated from Clusia burle-marxii
Abstract
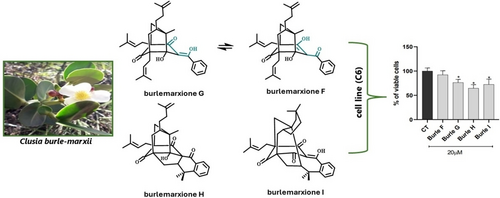
Three new polyprenylated benzophenone derivatives named burlemarxiones G−I (1-3) were isolated from C. burle-marxii trunks (compound 1) and leaves (compounds 2 and 3), along with the known compound burlemarxione F. Burlemarxione G (1) was isolated after methylation with diazomethane and it is the keto-enol tautomeric pair of burlemarxione F. Burlemarxione H (2) derives from burlemarxiones F and G, but it has additional rings due to cyclization of the prenyl group attached to C-5 that establishes new single bonds between C-1 and C-23, as well as, between C-24 and C-29. Burlemarxione I (3) has two additional cyclizations: the first encompasses the cyclization of the former isopentenyl group into an 11,11-dimethyl-six-membered ring, whereas the second produces additional rings due to the cyclization of the prenyl group attached to C-5 that establishes new single bonds between C-1 and C-23, as well as, between C-24 and C-29. All three compounds showed moderate anti-glioma activity. These results show that C. burle-marxii is an important source of sophisticated polyprenylated benzophenone derivatives.
Graphical Abstract
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.