Selective Activity Against Amastigote Forms of Trypanosoma cruzi and Leishmania infantum of Diastereomeric Dicentrine N-oxides
Abstract
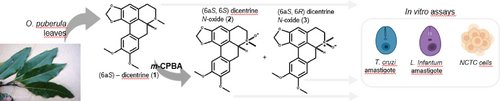
As part of our continuous research for the discovery of bioactive compounds against Trypanosoma cruzi and Leishmania infantum, the alkaloid (6aS)-dicentrine (1) was oxidized to afford (6aS,6S)- (2) and (6aS,6R)- (3) dicentrine-N-oxides. Evaluation of the cytotoxicity against NCTC cells indicated that 2 and 3 are non-toxic (CC50>200 μM) whereas 1 demonstrated CC50 of 52.0 μM. Concerning T. cruzi activity against amastigotes, derivatives 2 and 3 exhibited EC50 values of 9.9 μM (SI>20.2) and 27.5 μM (SI>7.3), respectively, but 1 is inactive (EC50>100 μM). Otherwise, when tested against L. infantum amastigotes, 1 and 3 exhibited EC50 values of 10.3 μM (SI=5.0) and 12.7 μM (SI>15.7), respectively, being 2 inactive (EC50>100 μM). Comparing the effects of positive controls benznidazol (EC50=6.5 μM and SI>30.7) and miltefosine (EC50=10.2 μM and SI=15.2), it was observed a selective antiparasitic activity to diastereomers 2 and 3 against T. cruzi and L. infantum. Considering stereochemical aspects, it was suggested that the configuration of the new stereocenter formed after oxidation of 1 played an important role in the bioactivity against amastigotes of both tested parasites.
Graphical Abstract
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.