The Sources, Structures and Cytotoxicity of Animal-Derived Bisindole Compounds
Zi-Long Zhang
School of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203 P.R. China
School of Pharmacy, Shaanxi Key Laboratory of Research and Application of “Taibai Qi Yao”, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi, 712046 P.R. China
Search for more papers by this authorHao-Nan Xu
School of Pharmacy, Shaanxi Key Laboratory of Research and Application of “Taibai Qi Yao”, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi, 712046 P.R. China
Search for more papers by this authorChuan-Ming Gong
School of Pharmacy, Shaanxi Key Laboratory of Research and Application of “Taibai Qi Yao”, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi, 712046 P.R. China
Search for more papers by this authorDr. Yu-Ze Li
School of Pharmacy, Shaanxi Key Laboratory of Research and Application of “Taibai Qi Yao”, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi, 712046 P.R. China
Search for more papers by this authorProf. Dr. Yi-Ming Li
School of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203 P.R. China
Search for more papers by this authorProf. Dr. Xiao-Mei Song
School of Pharmacy, Shaanxi Key Laboratory of Research and Application of “Taibai Qi Yao”, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi, 712046 P.R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Rui Wang
School of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203 P.R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Dong-Dong Zhang
School of Pharmacy, Shaanxi Key Laboratory of Research and Application of “Taibai Qi Yao”, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi, 712046 P.R. China
Search for more papers by this authorZi-Long Zhang
School of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203 P.R. China
School of Pharmacy, Shaanxi Key Laboratory of Research and Application of “Taibai Qi Yao”, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi, 712046 P.R. China
Search for more papers by this authorHao-Nan Xu
School of Pharmacy, Shaanxi Key Laboratory of Research and Application of “Taibai Qi Yao”, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi, 712046 P.R. China
Search for more papers by this authorChuan-Ming Gong
School of Pharmacy, Shaanxi Key Laboratory of Research and Application of “Taibai Qi Yao”, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi, 712046 P.R. China
Search for more papers by this authorDr. Yu-Ze Li
School of Pharmacy, Shaanxi Key Laboratory of Research and Application of “Taibai Qi Yao”, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi, 712046 P.R. China
Search for more papers by this authorProf. Dr. Yi-Ming Li
School of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203 P.R. China
Search for more papers by this authorProf. Dr. Xiao-Mei Song
School of Pharmacy, Shaanxi Key Laboratory of Research and Application of “Taibai Qi Yao”, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi, 712046 P.R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Rui Wang
School of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203 P.R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Dong-Dong Zhang
School of Pharmacy, Shaanxi Key Laboratory of Research and Application of “Taibai Qi Yao”, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi, 712046 P.R. China
Search for more papers by this authorAbstract
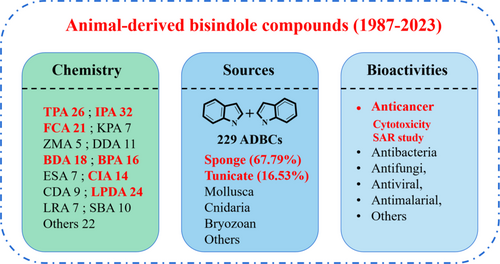
Bisindole compounds constitute a significant class of natural compounds distinguished by their characteristic bisindole structure and renowned for their anticancer properties. Over the past four decades, researchers have isolated 229 animal-derived bisindole compounds (ADBCs) from various animals. These compounds demonstrate a wide range of pharmacological properties, including cytotoxicity, antibacterial, antifungal, antiviral, and other activities. Notably, among these activities, cytotoxicity emerges as the most prominent characteristic of ADBCs. This review also summarizes the structureactivity relationship (SAR) studies associated with the cytotoxicity of these compounds and explores the druggability of these compounds. In summary, our objective is to provide an overview of the research progress concerning ADBCs, with the aim of fostering their continued development and utilization.
Graphical Abstract
Conflict of Interests
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv202401165-sup-0001-misc_information.pdf246.5 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1R. L. Siegel, K. D. Miller, N. S. Wagle, A. Jemal, CA Cancer J. Clin. 2023, 73, 17–48.
- 2
- 2aW. Tian, J. Huang, W. Zhang, Y. Wang, R. Jin, H. Guo, Y. Tang, Y. Wang, H. Lai, E. L.-H. Leung, Pharmacol. Res. 2024, 199, 107034;
- 2bM. D. W. Adico, B. Bayala, J. Bunay, S. Baron, J. Simpore, J.-M. A. Lobaccaro, Pharmacol. Res. 2024, 202, 107138.
- 3
- 3aY. Zhang, C. Hu, Arch. Pharm. 2020, 353, 2000092;
- 3bM. Xu, Z. Bai, B. Xie, R. Peng, Z. Du, Y. Liu, G. Zhang, S. Yan, X. Xiao, S. Qin, Molecules 2024, 29, 933.
- 4
- 4aD. S.-Y. Sim, S. Navanesan, K.-S. Sim, S. Gurusamy, S.-H. Lim, Y.-Y. Low, T.-S. Kam, J. Nat. Prod. 2019, 82, 850–858;
- 4bA. E. Nugroho, Y. Ono, E. Jin, Y. Hirasawa, T. Kaneda, A. Rahman, I. Kusumawati, T. Tougan, T. Horii, N. C. Zaini, H. Morita, J. Nat. Med. 2021, 75, 408–414.
- 5
- 5aS. Shaker, R.-Z. Fan, H.-J. Li, W.-J. Lan, Nat. Prod. Res. 2021, 35, 1497–1503;
- 5bW. Zhang, Z. Liu, S. Li, T. Yang, Q. Zhang, L. Ma, X. Tian, H. Zhang, C. Huang, S. Zhang, J. Ju, Y. Shen, C. Zhang, Org. Lett. 2012, 14, 3364–3367.
- 6D. J. Kliebenstein, Science 2018, 361, 642–643.
- 7
- 7aT. Yacoub, M. Rima, M. Karam, J.-M. Sabatier, Z. Fajloun, Molecules 2020, 25, 2402;
- 7bC. Laguionie-Marchais, A. L. Allcock, B. J. Baker, E.-A. Conneely, S. G. Dietrick, F. Kearns, K. McKeever, R. M. Young, C. A. Sierra, S. Soldatou, H. L. Woodcock, M. P. Johnson, Mar. Drugs 2022, 20, 42;
- 7cS. Y. Park, H. Choi, J. W. Chung, Pharmaceutics 2022, 14, 874.
- 8
- 8aX. Bai, Y. Liu, H. Wang, H. Zhang, Molecules 2021, 26, 1097;
- 8bL. Chen, J.-S. Hu, J.-L. Xu, C.-L. Shao, G.-Y. Wang, Mar. Drugs 2018, 16, 362.
- 9N. L. Segraves, S. J. Robinson, D. Garcia, S. A. Said, X. Fu, F. J. Schmitz, H. Pietraszkiewicz, F. A. Valeriote, P. Crews, J. Nat. Prod. 2004, 67, 783–792.
- 10P. C. Jimenez, D. V. Wilke, E. G. Ferreira, R. Takeara, M. O. De Moraes, E. R. Silveira, T. M. Da Cruz Lotufo, N. P. Lopes, L. V. Costa-Lotufo, Mar. Drugs 2012, 10, 1092–1102.
- 11
- 11aS.-F. Teng, F.-R. Li, Q.-M. Cui, A. Khan, T. He, X.-D. Luo, Y.-P. Liu, G.-G. Cheng, Phytochem. Rev. 2023, 22, s11101-023-09871-2;
- 11bS. Kumar, B. Singh, R. Singh, J. Ethnopharmacol. 2022, 284, 114647;
- 11cA. Mauger, M. Jarret, C. Kouklovsky, E. Poupon, L. Evanno, G. Vincent, Nat. Prod. Rep. 2021, 38, 1852–1886;
- 11dH. Yang, H. Zhang, C. Yang, Y. Chen, Chem. Biodivers. 2016, 13, 807–820;
- 11eY.-G. Chen, J.-C. Wu, G.-Y. Chen, C.-R. Han, X.-P. Song, Chem. Biodivers. 2011, 8, 1958–1967
- 12J. A. Homer, J. Sperry, J. Nat. Prod. 2017, 80, 2178–2187.
- 13K. Bartik, J.-C. Braekman, D. Daloze, C. Stoller, J. Huysecom, G. Vandevyver, R. Ottinger, J. Chem. 1987, 65, 2118–2121.
- 14S. Tsujii, K. L. Rinehart, S. P. Gunasekera, Y. Kashman, S. S. Cross, M. S. Lui, S. A. Pomponi, M. C. Diaz, J. Org. Chem. 1988, 53, 5446–5453.
- 15L. Murray, T. Lim, J. Hooper, R. Capon, Aust. J. Chem. 1995, 48, 2053–2058.
- 16A. Casapullo, G. Bifulco, I. Bruno, R. Riccio, J. Nat. Prod. 2000, 63, 447–451.
- 17B. Bao, Q. Sun, X. Yao, J. Hong, C.-O. Lee, C. J. Sim, K. S. Im, J. H. Jung, J. Nat. Prod. 2005, 68, 711–715.
- 18B. Bao, Q. Sun, X. Yao, J. Hong, C.-O. Lee, H. Y. Cho, J. H. Jung, J. Nat. Prod. 2007, 70, 2–8.
- 19S. A. Morris, R. J. Andersen, Tetrahedron 1990, 46, 715–720.
- 20H.-B. Liu, G. Lauro, R. D. O'Connor, K. Lohith, M. Kelly, P. Colin, G. Bifulco, C. A. Bewley, J. Nat. Prod. 2017, 80, 2556–2560.
- 21J. S. Park, E. Cho, J.-Y. Hwang, S. C. Park, B. Chung, O.-S. Kwon, C. J. Sim, D.-C. Oh, K.-B. Oh, J. Shin, Mar. Drugs 2021, 19.
- 22K.-B. Oh, W. Mar, S. Kim, J.-Y. Kim, T.-H. Lee, J.-G. Kim, D. Shin, C. J. Sim, J. Shin, Biolog. Pharmaceut. Bull. 2006, 29, 570–573.
- 23N. A. Khan, N. Kaur, P. Owens, O. P. Thomas, A. Boyd, Int. J. Molecular Sci. 2022, 23, 13774.
- 24H. Sato, M. Tsuda, K. Watanabe, J. i. Kobayashi, Tetrahedron 1998, 54, 8687–8690.
- 25S. Kohmoto, Y. Kashman, O. J. McConnell, K. L. Rinehart, Jr., A. Wright, F. Koehn, J. Org. Chem. 1988, 53, 3116–3118.
- 26P. G. Cruz, J. F. Martínez Leal, A. H. Daranas, M. Pérez, C. Cuevas, ACS Omega 2018, 3, 3760–3767.
- 27E. Fahy, B. C. M. Potts, D. J. Faulkner, K. Smith, J. Nat. Prod. 1991, 54, 564–569.
- 28A. E. Wright, S. A. Pomponi, S. S. Cross, P. McCarthy, J. Org. Chem. 1992, 57, 4772–4775.
- 29S. P. Gunasekera, P. J. McCarthy, M. Kelly-Borges, J. Nat. Prod. 1994, 57, 1437–1441.
- 30R. J. Capon, F. Rooney, L. M. Murray, E. Collins, A. T. R. Sim, J. A. P. Rostas, M. S. Butler, A. R. Carroll, J. Nat. Prod. 1998, 61, 660–662.
- 31A. Cutignano, G. Bifulco, I. Bruno, A. Casapullo, L. Gomez-Paloma, R. Riccio, Tetrahedron 2000, 56, 3743–3748.
- 32L. K. Jennings, N. M. D. Khan, N. Kaur, D. Rodrigues, C. Morrow, A. Boyd, O. P. Thomas, Molecules 2019, 24, 3890.
- 33C. Jimenez, E. Quinoa, M. Adamczeski, L. M. Hunter, P. Crews, J. Org. Chem. 1991, 56, 3403–3410.
- 34S. Ankietty, M. Kelly, M. Slattery, Nat. Prod. Commun. 2007, 2, 1145–1148 https://doi.org.1934578X0700201120.
- 35S. Khokhar, Y. Feng, M. R. Campitelli, M. G. Ekins, J. N. A. Hooper, K. D. Beattie, M. C. Sadowski, C. C. Nelson, R. A. Davis, Bioorg. Med. Chem. Lett. 2014, 24, 3329–3332.
- 36Z. Lu, Y. Ding, X.-C. Li, D. R. Djigbenou, B. T. Grimberg, D. Ferreira, C. M. Ireland, R. M. Van Wagoner, Bioorg. Med. Chem. 2011, 19, 6604–6607.
- 37K. M. Meragelman, L. M. West, P. T. Northcote, L. K. Pannell, T. C. McKee, M. R. Boyd, J. Org. Chem. 2002, 67, 6671–6677.
- 38F. Russell, D. Harmody, P. J. McCarthy, S. A. Pomponi, A. E. Wright, J. Nat. Prod. 2013, 76, 1989–1992.
- 39T. Sasaki, I. I. Ohtani, J. Tanaka, T. Higa, Tetrahedron Lett. 1999, 40, 303–306.
- 40B. K. S. Yeung, Y. Nakao, R. B. Kinnel, J. R. Carney, W. Y. Yoshida, P. J. Scheuer, M. Kelly-Borges, J. Org. Chem. 1996, 61, 7168–7173.
- 41Y. Nakao, J. Kuo, W. Y. Yoshida, M. Kelly, P. J. Scheuer, Org. Lett. 2003, 5, 1387–1390.
- 42K. A. El Sayed, M. Kelly, U. A. K. Kara, K. K. H. Ang, I. Katsuyama, D. C. Dunbar, A. A. Khan, M. T. Hamann, J. Am. Chem. Soc. 2001, 123, 1804–1808.
- 43Y. Takahashi, T. Kubota, J. Fromont, J. i. Kobayashi, Org. Lett. 2009, 11, 21–24.
- 44M. Yamada, Y. Takahashi, T. Kubota, J. Fromont, A. Ishiyama, K. Otoguro, H. Yamada, S. Ōmura, J. i. Kobayashi, Tetrahedron 2009, 65, 2313–2317.
- 45T. Kubota, K. Nakamura, S.-i. Kurimoto, K. Sakai, J. Fromont, T. Gonoi, J. i. Kobayashi, J. Nat. Prod. 2017, 80, 1196–1199.
- 46G. Bifulco, I. Bruno, R. Riccio, J. Lavayre, G. Bourdy, J. Nat. Prod. 1995, 58, 1254–1260.
- 47P.-E. Campos, E. Pichon, C. Moriou, P. Clerc, R. Trépos, M. Frederich, N. De Voogd, C. Hellio, A. Gauvin-Bialecki, A. Al-Mourabit, Mar. Drugs 2019, 17, 167.
- 48P. Neupane, A. A. Salim, R. J. Capon, Tetrahedron Lett. 2020, 61, 151651.
- 49J. Dai, J. I. Jiménez, M. Kelly, S. Barnes, P. Lorenzo, P. Williams, J. Nat. Prod. 2008, 71, 1287–1290.
- 50J. Dai, J. I. Jiménez, M. Kelly, P. G. Williams, J. Org. Chem. 2010, 75, 2399–2402.
- 51T. Iwagawa, M. Miyazaki, Y. Yokogawa, J. Heterocycles 2008, 75, 2023–2028.
- 52M. Meyer, F. Delberghe, F. Liron, M. Guillaume, A. Valentin, M. Guyot, Nat. Prod. Res. 2009, 23, 178–182.
- 53I. Mancini, G. Guella, H. Zibrowius, F. Pietra, Tetrahedron 2003, 59, 8757–8762.
- 54P. A. Horton, R. E. Longley, O. J. McConnell, L. M. Ballas, Experientia 1994, 50, 843–845.
- 55R. B. Kinnel, P. J. Scheuer, J. Organ. Chem. 1992, 57, 6327–6329.
- 56P. Schupp, C. Eder, P. Proksch, V. Wray, B. Schneider, M. Herderich, V. Paul, J. Nat. Prod. 1999, 62, 959–962.
- 57P. Schupp, P. Proksch, V. Wray, J. Nat. Prod. 2002, 65, 295–298.
- 58M. Tadesse, J. N. Tabudravu, M. Jaspars, M. B. Strøm, E. Hansen, J. H. Andersen, P. E. Kristiansen, T. Haug, J. Nat. Prod. 2011, 74, 837–841.
- 59J. C. Swersey, C. M. Ireland, L. M. Cornell, R. W. Peterson, J. Nat. Prod. 1994, 57, 842–845.
- 60D. M. Tapiolas, B. F. Bowden, E. Abou-Mansour, R. H. Willis, J. R. Doyle, A. N. Muirhead, C. Liptrot, L. E. Llewellyn, C. W. W. Wolff, A. D. Wright, C. A. Motti, J. Nat. Prod. 2009, 72, 1115–1120.
- 61M. Salmoun, C. Devijver, D. Daloze, J.-C. Braekman, R. W. M. van Soest, J. Nat. Prod. 2002, 65, 1173–1176.
- 62D. T. A. Youssef, J. Nat. Prod. 2005, 68, 1416–1419.
- 63E. G. Lyakhova, S. A. Kolesnikova, A. I. Kalinovsky, S. S. Afiyatullov, S. A. Dyshlovoy, V. B. Krasokhin, C. V. Minh, V. A. Stonik, Tetrahedron Lett. 2012, 53, 6119–6122.
- 64D. T. A. Youssef, L. A. Shaala, H. Z. Asfour, Mar. Drugs 2013, 11, 1061–1070.
- 65A. Badre, A. Boulanger, E. Abou-Mansour, B. Banaigs, G. Combaut, C. Francisco, J. Nat. Prod. 1994, 57, 528–533.
- 66S.-I. Park, Y.-J. Lee, H. Won, K.-B. Oh, H.-S. Lee, Nat. Prod. Commun. 2018, 13, 683–685https://doi.org/10.1177/1934578X1801300608.
- 67D. John Faulkner, Nat. Prod. Rep. 1998, 15, 113–158.
- 68T.-Y. Jin, P.-L. Li, C.-L. Wang, X.-L. Tang, M.-M. Cheng, Y. Zong, L.-Z. Luo, H.-L. Ou, K.-C. Liu, G.-Q. Li, Chin. J. Chem. 2021, 39, 2588–2598.
- 69S. A. Abas, M. B. Hossain, D. van der Helm, F. J. Schmitz, M. Laney, R. Cabuslay, R. C. Schatzman, J. Org. Chem. 1996, 61, 2709–2712.
- 70C. Moquin-Pattey, M. Guyot, Tetrahedron 1989, 45, 3445–3450.
- 71P. S. Kearns, J. C. Coll, J. A. Rideout, J. Nat. Prod. 1995, 58, 1075–1076.
- 72P. S. Kearns, J. A. Rideout, J. Nat. Prod. 2008, 71, 1280–1282.
- 73G. Kleks, S. Duffy, L. Lucantoni, V. M. Avery, A. R. Carroll, J. Nat. Prod. 2020, 83, 422–428.
- 74J. S. Sandler, P. L. Colin, J. N. A. Hooper, D. J. Faulkner, J. Nat. Prod. 2002, 65, 1258–1261.
- 75K. Ragini, A. M. Piggott, P. Karuso, Mar. Drugs 2019, 17, 683.
- 76D. R. Appleton, R. C. Babcock, B. R. Copp, Tetrahedron 2001, 57, 10181–10189.
- 77H. R. Bokesch, L. K. Pannell, T. C. McKee, M. R. Boyd, Tetrahedron Lett. 2000, 41, 6305–6308.
- 78D. Wang, Y. Feng, M. Murtaza, S. Wood, G. Mellick, J. N. A. Hooper, R. J. Quinn, J. Nat. Prod. 2016, 79, 353–361.
- 79R. Momose, N. Tanaka, J. Fromont, J. i. Kobayashi, Org. Lett. 2013, 15, 2010–2013.
- 80N. Tanaka, R. Momose, A. Takahashi-Nakaguchi, T. Gonoi, J. Fromont, J. i. Kobayashi, Tetrahedron 2014, 70, 832–837.
- 81P. Sauleau, M.-T. Martin, M.-E. T. H. Dau, D. T. A. Youssef, M.-L. Bourguet-Kondracki, J. Nat. Prod. 2006, 69, 1676–1679.
- 82T. Kubota, K. Nakamura, K. Sakai, J. Fromont, T. Gonoi, J. i. Kobayashi, Chem. Pharmaceut. Bull. 2016, 64, 975–978.
- 83P. E. McGovern, R. H. Michel, Acc. Chem. Res. 1990, 23, 152–158.
- 84W. P. Kelley, A. M. Wolters, J. T. Sack, R. A. Jockusch, J. C. Jurchen, E. R. Williams, J. V. Sweedler, W. F. Gilly, J. Biol. Chem. 2003, 278, 34934–34942.
- 85L. Meijer, A. L. Skaltsounis, P. Magiatis, P. Polychronopoulos, M. Knockaert, M. Leost, X. P. Ryan, C. A. Vonica, A. Brivanlou, R. Dajani, C. Crovace, C. Tarricone, A. Musacchio, S. M. Roe, L. Pearl, P. Greengard, Chem. Biol. 2003, 10, 1255–1266.
- 86T. Abe, A. Kukita, K. Akiyama, T. Naito, D. Uemura, Chem. Lett. 2012, 41, 728–729.
- 87M. Tsuda, Y. Takahashi, J. Fromont, Y. Mikami, J. i. Kobayashi, J. Nat. Prod. 2005, 68, 1277–1278.
- 88N. Hanif, K. Yamada, M. Kitamura, Y. Kawazoe, N. J. de Voogd, D. Uemura, Chem. Nat. Compd. 2015, 51, 1130–1133.
- 89A. G. Guzii, T. N. Makarieva, S. N. Fedorov, V. A. Denisenko, P. S. Dmitrenok, A. S. Kuzmich, V. B. Krasokhin, H.-S. Lee, Y.-J. Lee, V. A. Stonik, 2016, 11.
- 90I. Mancini, G. Guella, F. Pietra, C. Debitus, J. Waikedre, Helvetica Chim. Acta 1996, 79, 2075–2082.
- 91S. J. Rochfort, S. Moore, C. Craft, N. H. Martin, R. M. Van Wagoner, J. L. C. Wright, J. Nat. Prod. 2009, 72, 1773–1781.
- 92T. Endo, M. Tsuda, J. Fromont, J. i. Kobayashi, J. Nat. Prod. 2007, 70, 423–424.
- 93Y.-J. Lee, D.-G. Lee, H. S. Rho, V. B. Krasokhin, H. J. Shin, J. S. Lee, H.-S. Lee, J. Heterocyclic Chem. 2013, 50, 1400–1404.
- 94H. C. Brastianos, E. Vottero, B. O. Patrick, R. Van Soest, T. Matainaho, A. G. Mauk, R. J. Andersen, J. Am. Chem. Soc. 2006, 128, 16046–16047.
- 95Y. Hitora, K. Takada, Y. Ise, S. Okada, S. Matsunaga, J. Nat. Prod. 2016, 79, 2973–2976.
- 96T. D. Tran, L. K. Cartner, H. R. Bokesch, C. J. Henrich, X. W. Wang, C. Mahidol, S. Ruchirawat, P. Kittakoop, B. R. O'Keefe, K. R. Gustafson, Magn. Reson. Chem. 2021, 59, 534–539.
- 97P. Moosmann, T. Taniguchi, K. Furihata, H. Utsumi, Y. Ise, Y. Morii, N. Yamawaki, T. Takatani, O. Arakawa, S. Okada, S. Matsunaga, Org. Lett. 2021, 23, 3477–3480.
- 98N. Tanaka, R. Momose, Y. Takahashi, T. Kubota, A. Takahashi-Nakaguchi, T. Gonoi, J. Fromont, J. i. Kobayashi, Tetrahedron Lett. 2013, 54, 4038–4040.
- 99X. Di, S. Wang, J. T. Oskarsson, C. Rouger, D. Tasdemir, I. Hardardottir, J. Freysdottir, X. Wang, T. F. Molinski, S. Omarsdottir, J. Nat. Prod. 2020, 83, 2854–2866.
- 100S. Sakemi, H. H. Sun, J. Org. Chem. 1991, 56, 4304–4307.
- 101J. i. Kobayashi, T. Murayama, M. Ishibashi, S. Kosuge, M. Takamatsu, Y. Ohizumi, H. Kobayashi, T. Ohta, S. Nozoe, S. Takuma, Tetrahedron 1990, 46, 7699–7702.
- 102M. J. McKay, A. R. Carroll, R. J. Quinn, J. N. A. Hooper, J. Nat. Prod. 2002, 65, 595–597.
- 103F. Malard, M. Mohty, Lancet 2020, 395, 1146–1162.
- 104
- 104aL. Scarfò, A. J. Ferreri, P. Ghia, Crit. Rev. Oncol./Hematology 2016, 104, 169–182;
- 104bS. Saleem, J. Amin, M. Sharif, G. A. Mallah, S. Kadry, A. H. Gandomi, Comput. Biol. Med. 2022, 150, 106028.
- 105A. Leiter, R. R. Veluswamy, J. P. Wisnivesky, Nat. Rev. Clin. Oncol. 2023, 20, 624–639.
- 106A. A. Thai, B. J. Solomon, L. V. Sequist, J. F. Gainor, R. S. Heist, Lancet (London, England) 2021, 398, 535–554.
- 107S. Jonna, D. S. Subramaniam, Disc. Med. 2019, 27, 167–170.
- 108E. C. Smyth, M. Nilsson, H. I. Grabsch, N. C. van Grieken, F. Lordick, Lancet (London, England) 2020, 396, 635–648.
- 109S. Lheureux, C. Gourley, I. Vergote, A. M. Oza, Lancet (London, England) 2019, 393, 1240–1253.
- 110P. P. Centeno, V. Pavet, R. Marais, Nat. Rev. Cancer 2023, 23, 372–390.
- 111C. Peng, X. Li, W. Tang, W. Zhu, P. Yan, J. Chen, X. Zhang, Q. Guo, Q. Wu, Q. Wang, N. Liu, A. Ma, Y. Lu, P. Lv, J. Liu, P. Xie, Int. Immunopharmacol. 2024, 129, 111578.
- 112P. A. Cohen, A. Jhingran, A. Oaknin, L. Denny, Lancet (London England) 2019, 393, 169–182.
- 113S. Turajlic, C. Swanton, C. Boshoff, J. Exp. Med. 2018, 215, 2477–2479.
- 114L. Zhu, Y. Chen, C. Wei, X. Yang, J. Cheng, Z. Yang, C. Chen, Z. Ji, Nat. Prod. Res. 2018, 32, 493–497.
- 115U. Veronesi, P. Boyle, A. Goldhirsch, R. Orecchia, G. Viale, Lancet (London England) 2005, 365, 1727–1741.
- 116E. Dekker, P. J. Tanis, J. L. A. Vleugels, P. M. Kasi, M. B. Wallace, Lancet (London, England) 2019, 394, 1467–1480.
- 117K. Thanikachalam, G. Khan, Nutrients 2019, 11, 164.
- 118A. C. Tan, D. M. Ashley, G. Y. López, M. Malinzak, H. S. Friedman, M. Khasraw, CA Cancer J. Clinicians 2020, 70, 299–312.
- 119
- 119aR. J. A. Goodwin, J. Bunch, D. F. McGinnity, in Advances in Cancer Research (Eds: R. R. Drake, L. A. McDonnell), Vol. 134, Academic Press 2017, pp. 133–171;
- 119bC. A. Lipinski, F. Lombardo, B. W. Dominy, P. J. Feeney, Adv. Drug Delivery Rev. 2001, 46, 3–26.
- 120C. Jiménez, ACS Med. Chem. Lett. 2018, 9, 959–961.
- 121E. S. Kim, Drugs 2017, 77, 1251–1259.
- 122M. Huang, H. S. Lin, Y. S. Lee, P. C. Ho, Int. J. Oncol. 2014, 45, 1724–1734.
- 123T. Bourhill, A. Narendran, R. N. Johnston, Crit. Rev. Oncol./Hematol. 2017, 112, 72–79.
- 124H. Alonso, A. A. Bliznyuk, J. E. Gready, Med. Res. Rev. 2006, 26, 531–568.
- 125J. Zhou, R. Zhang, H. Yan, X. Liu, C. Shang, Y. Chen, Nat. Prod. Res. 2024, 38, 1–4.
- 126C. A. Lipinski, Adv. Drug Delivery Rev. 2016, 101, 34–41.
- 127H. Yao, J. Liu, S. Xu, Z. Zhu, J. Xu, Expert Opin. Drug Discovery 2017, 12, 121–140.
- 128Y. Chen, S. Wang, Q. Hu, L. Zhou, Curr. Drug Delivery 2023, 20, 919–926.
- 129
- 129aY. Chen, Z. Zhang, Z. Qian, R. Ma, M. Luan, Y. Sun, Int. J. Nanomed. 2024, 19, 727–742;
- 129bD. Liu, T. Hou, C. Geng, L. Song, X. Hou, Y. Chen, F. Wang, W. Wang, B. Han, L. Gao, J. Pharmaceut. Sci. 2024, 113, 1572–1579.
- 130P. Saokham, C. Muankaew, P. Jansook, T. Loftsson, Molecules 2018, 23, 42.
10.3390/molecules23051161 Google Scholar