Synthesis of Para-Acetylated Functionalized Ni(II)-POCOP Pincer Complexes and Their Cytotoxicity Evaluation Against Human Cancer Cell Lines
Abstract
A series of three Ni(II)-POCOP complexes para-functionalized with an acetoxyl fragment were synthesized. All complexes (2 a–c) were fully characterized through standard analytical techniques. The molecular structure of complex 2 b was unambiguously determined by single-crystal X-ray diffraction, revealing that the metal center is situated in a slightly distorted square-planar environment. Additionally, the acetoxy fragment at the para-position of the phenyl ring was found to be present. The in vitro cytotoxic activity of all complexes was assessed on six human cancer cell lines. Notably, complex 2 b exhibited selective activity against K-562 (chronic myelogenous leukemia) and MCF-7 (mammary adenocarcinoma) with IC50 values of 7.32±0.60 μM and 14.36±0.02 μM, respectively. Furthermore, this compound showed negligible activity on the healthy cell line COS-7, highlighting the potential therapeutic application of 2 b. The cytotoxic evaluations were further complemented with molecular docking calculations to explore the potential biological targets of complex 2 b, revealing interactions with cluster differentiation protein 1a (CD1 A, PDB: 1xz0) for K-562 and with the progesterone receptor for MCF-7.
Graphical Abstract
1 Introduction
Cancer disease is caused by an irregular cell growth with the ability to be spread throughout the organism and invade healthy tissue.1, 2 According to the World Health Organization's estimation in 2019, cancer stands as the second leading cause of death, impacting millions of people worldwide. Its profound effects are felt not only at the individual level but also across societies.3 The diverse classes of cancer, such as carcinomas, lymphomas, sarcomas, or leukemia requires a specific treatment that currently involve the use of surgery or radiation therapy. However, there is still an urgent need for more effective, selective, and less toxic therapies. Since the discovery of cisplatin and its successful application as an anticancer agent, research on metallodrugs has been growing constantly.4 Although platinum-based metallodrugs are currently employed in chemotherapy, their limited ability to target specific cancers, coupled with the side effects such as drug resistance, nephrotoxicity, and hepatotoxicity, has encouraged the development of a new class of non-platinum anticancer metallodrugs.
Nickel is a trace element with biological roles in humans directly related to the absorption of iron in the body, participation as a cofactor in enzymes, and acting as a mediator in the production of prolactin in lactating women.5 Although nickel and its derivatives were classified as human carcinogens by the World Health Organization in 1990,6 its biological importance is gradually being recognized.7 Diverse studies8, 9 have demonstrated that nickel salts, with the exception of nickel disulfide or nickel tetracarbonyl, do not cause cancer.10, 11 Therefore, nickel(II) has received significant attention as an anticancer agent owing to its unique coordination chemistry, redox properties, and its ability to cross-link DNA, similar to platinum(II).2 Recently, it has been reported that nickel complexes based on Schiff-base ligands12 exhibit excellent cytotoxicity against various cancer cell lines, including lung, breast, and ovarian cancer cells. Consequently, exploring the cytotoxic activity of other nickel compounds, such as those incorporating pincer ligands, is pertinent for expanding the study of compounds with anticancer properties.
Pincer complexes (Figure 1) are a class of organometallic compounds that feature a ligand with a pair of donor atoms (D) in their structure capable of coordinating in a tridentate manner.13 The donors include several examples such as PR2, P(OR)2, NR2, OR, or SR, which have “side arms”. These side arms are useful for stabilizing the structure and modulating the electronic properties of the complex.14 The ′Z’ atoms between the donors (D) and the aryl moiety are known as spacers, and their modification has led to pincer systems, such as POCOP complexes when D is oxygen.15 Pincer structures not only enhance the stability of metal-coordination compounds against biological reduction or redox reactions but also combine the characteristics of metallic centers and organic scaffolds.16
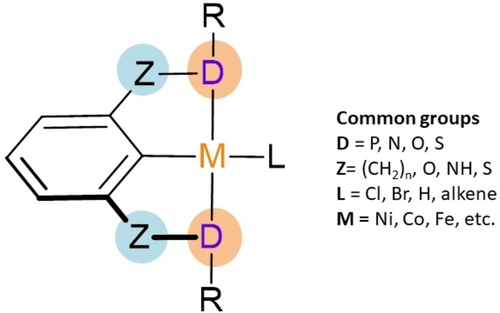
General structure of a pincer complex.
In this context, pincer compounds based on first-row transition metals have demonstrated potential anticancer activity against important cell lines such as HeLa, HepG-2, MCF-7, and Bel-7402.17-21 The different properties of the metal centers appear to provide various pharmacological targets, which are expected to overcome drug resistance. On the other hand, the coordinated ligands also play a crucial role in the anticancer activity of metallodrugs, as they can modify key parameters such as reactivity and lipophilicity. Importantly, in the pincer complexes, the aromatic central structure and their side arms can be functionalized with specific moieties to modify biological activity.22 In this regard, it has been reported that acetylated motifs in certain drugs enhance their solubility, facilitate drug delivery, and improve aqueous stability in physiological media.23, 24 For instance, the acetylation of the antibiotic chloramphenicol increases its potency against a wide range of bacteria. Similarly, the acetylation of the antiviral drug ribavirin enhances its activity against hepatitis C virus.25 Based on the evidence previously presented, in this work we report the synthesis of three para-functionalized nickel (II) POCOP pincer complexes and their preliminary in vitro cytotoxic in six human cancer cell-lines. We hypothesized that incorporating an acetoxy fragment in the pincer ligand could enhance the solubility, cytotoxicity, stability, and bioavailability of these complexes.
Materials and Methods
Materials
All complexes were prepared under a nitrogen closed system using standard Schlenk techniques. All commercially acquired reagents were used without further purification. The solvents utilized in the synthesis were dried with metallic sodium and distilled under a nitrogen atmosphere. The 1H, 13C{1H} and 31P{1H} NMR spectra were recorded on a Bruker Ascend 700 spectrometer or on a JEOL GX300 spectrometer, using CDCl3 as a solvent for the complexes. Chemical shifts are reported in ppm down field of TMS using the residual signals in the solvent (CDCl3, δ 7.26 ppm) as internal standard. Mass spectra were acquired on a MStation JMS-700 Mass Spectrometer. Elemental analyses were performed on a Perkin Elmer 240. CHNS analyses were performed in Thermo Scientific Flash 2000 elemental analyzed using a Mettler Toledo XP6 Automated-S Microbalance and sulfanilamide as standard (Thermo Scientific BN 217826, attained values N=16.40 %, C=41.91 %, H=4.65 % and S=18.63 %; certified values N=16.26 %, C=41.81 %, H=4.71 % and S=18.62 %). Complex purity determination was conducted using an Alliance Waters E2695 chromatograph equipped with a photodiode array 2996 detector (254 nm), using a column Kinetex 5 mm EVO C18 100 Å 100x2.1 mm with a flow rate of 0.2 mL/min of the eluent MeCN: 0.1 % formic acid solution (80 : 20).
Synthesis of Para-Acetylated Functionalized Ni(II)-POCOP Pincer Complexes
Synthesis of Precursors Complexes 1 a–1 c
The para-hydroxi Ni(II)-POCOP pincer complexes 1 a–1 c, used as starting materials, were synthesized following a procedure previously reported by our group (Scheme 1).26 All compounds 1 a–1c were fully characterized by multinuclear NMR (1H and 31P{1H}), and all signals are consistent with those reported previously.
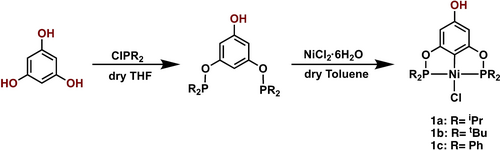
General procedure for the synthesis of precursors complexes 1 a–1 c
For 1 a ([NiCl{C6H2-4-OH-2,6-(OPiPr2)2}]): yellow crystalline solid, yield: 83 %, mp 152 °C. 1H-NMR (CDCl3, 25 °C, 300 MHz) δ: 1.3 (m, 24H), 2.33 (m, 4H), 5.93 (s, 2H). 13C{1H} NMR (CDCl3, 25 °C, 75 MHz) δ: 16.9, 17.6, 27.9, 94.0, 114.0, 157.9, 169.0. 31 P{1H} NMR (CDCl3, 25 °C, 122 MHz) δ: 187.34. EM (EI+; m/z): 452 [M]+. Elem. Anal. Calcd. for C18H31ClNiO3P2: C, 47.88; H, 6.92. Found: C, 47.91, H, 6.95 %.
For 1b ([NiCl{C6H2-4-OH-2,6-(OPtBu2)2}]): yellow crystalline solid, yield 89 %, mp 265 °C. 1H-NMR (CDCl3, 25 °C, 300 MHz) δ: 1.4 (m, 36H), 5.91 (s, 2H). 13C{1H} NMR (CDCl3, 25 °C, 75 MHz) δ: 27.8, 39.1, 93.7, 114.2, 157.9, 170.3. 31P{1H} NMR (CDCl3, 25 °C, 122 MHz) δ: 189.81. EM (FAB+; m/z): 508 [M]+. Elem. Anal. Calcd. for C22H39ClNiO3P2: C, 52.05; H, 7.74. Found: C, 51.98, H, 7.79 %.
For 1c ([NiCl{C6H2-4-OH-2,6-(OPPh2)2}]): yellow crystalline solid, yield 89 %, mp 326 °C. 1H-NMR (CDCl3, 25 °C, 300 MHz) δ: 6.9 (s, 2H), 7.2 (m, 20H). 13C{1H} NMR (CDCl3, 25 °C, 75 MHz). δ: 95.3, 128.3, 128.5, 128.9, 131.1, 131.6, 131.7, 132.5. 31P{1H} NMR (CDCl3, 25 °C, 122 MHz) δ: 147.7. EM (EI+; m/z): 588 [M]+. Elem. Anal. Calcd. for C30H23ClNiO3P2: C, 61.32; H, 3.95. Found: C, 61.27, H, 3.79 %.
Synthesis of 4-Acetoxy-POCOP-Ni(II), 2 a and 2 b
A mixture of the corresponding para-hydroxy Ni(II) POCOP pincer complex (1 eq.), K2CO3 (1.5 eq.), and acetyl chloride (1.1 eq.) in acetone was refluxed for 18 hours. After this period, the resulting solution was cooled to room temperature, filtered through celite, and evaporated under high vacuum. The crude products were purified by chromatographic column using an EtOAc/Hexane (2 : 8) mixture. The purified products were obtained as yellow solids.
For 2 a: [NiCl{C6H2-4-(OCOCH3)-2,6-(OP(iPr)2)2}]. The synthesis of (2 a) was performed using (1 a) (112.8 mg, 0.25 mmol). Yield: 89.1 mg (72.2 %). 1H-NMR (CDCl3, 25 °C, 700 MHz,) δ: 6.20 (s, 2H, 2 x CHAr), 2.44−2.38 (m, 4H, 4xCHiPr), 2.23 (s, 3H, −COCH3), 1.45−1.41 (m, 12H, 4x−CH3iPr), 1.35−1.32 (m, 12H, 4x−CH3iPr). 13C{1H} NMR (176 MHz, CDCl3) δ: 169.27 (C=O), 168.41 (t, 2JC-p=10.3 Hz, 2xCAr), 151.59 (CC-OAc), 121.82 (t, 2JC-P=21.8 Hz, CC-Ni), 99.49 (t, 3JC-P=6.3 Hz, 2xCHAr), 27.99 (t, 1JC-P=10.8 Hz, 4xCHiPr), 21.31 (−COCH3), 17.64 (4x−CH3iPr), 16.86 (4x−CH3iPr). 31P{1H} NMR (122 MHz, CDCl3) δ 187.03. MS (FAB+; m/z) 492 [M]+. Elem. Anal. Calcd. for C20H33ClNiO4P2: C, 48.67; H, 6.74. Found: C, 48.80; H, 6.68 %. HPLC-PDA purity: 96.98 %.
For 2 b: [NiCl{C6H2-4-(OCOCH3)-2,6-(OP(tBu)2)2}] (2 b). The synthesis of (2 b) was performed using (1b) (126.9 mg, 0.25 mmol). Yield: 96.7 mg (70.3 %). 1H-NMR (CDCl3, 500 MHz), δ: 6.20 (s, 2H, CHAr), 2.23 (s, 3H, −COCH3), 1.49−1.46 (m, 36H, 12x−CH3tBu). 13C{1H} NMR (126 MHz, CDCl3) δ: 169.29 (C=O), 168.99 (t,2JC-p=10.3 Hz, 2xCAr), 151.25 (CC-OAc), 121.27 (t, 2JC-P=21.1 Hz, CC-Ni), 99.04 (t, 3JC-P=6.0 Hz, 2xCHAr), 39.44 (t, 1JC-P=6.9 Hz, 4xCtBu), 28.10 (12x−CH3tBu), 21.32 (−COCH3). 31P{1H} NMR (CDCl3, 202 MHz) δ: 189.56. MS (FAB+; m/z) 548 [M]+. Elem. Anal. Calcd. for C20H33ClNiO4P2: C, 52.44; H, 7.52. Found: C, 52.61; H, 7.38 %. HPLC-PDA purity: 95.98 %.
Synthesis of 4-Acetoxy-POCOP-Ni(II), 2 c
A Schlenk flask containing a mixture of complex 1 c (117.5 mg, 0.2 mmol) and sodium tetramethoxyborate (63.1 mg, 0.4 mmol) in dry THF (10 mL) was stirred under a nitrogen atmosphere at room temperature for 2 hours. Then, 0.3 mmol of acetyl chloride was added, and the mixture continued stirring for an additional 18 hours. The resulting solution was cooled to room temperature, filtered through celite, and evaporated under high vacuum. The crude product was purified by chromatographic column using an EtOAc/Hexane (2 : 8) mixture. Compound 2 c was obtained as a yellow solid.
For 2 c ([NiCl{C6H2-4-(OCOCH3)-2,6-(OP(Ph)2)2}]): Yield: 70.4 mg (59.2 %). 1H-NMR (700 MHz, CDCl3) δ: 7.99−7.97 (m, 8H, CH, Ar), 7.53−7.46 (m, 12H, CH, Ph), 6.41 (s, 2H, CH, Ar), 2.26 (s, 3H, −COCH3). 13C{1H} NMR (176 MHz, CDCl3) δ: 169.36 (C=O), 166.90−166.77 (m, 2 C, Ar), 152.25 (s, C, Ar), 132.47−132.20 (m, C, Ph), 132.07 (s, C, Ph), 132.03−132.01 (m, 2CH, Ph), 128.97−128.92 (m, 2CH, Ph), 123.31−123.04 (m, C−Ni), 101.05−100.97 (m, 2 C, Ar), 21.24 (−COCH3). 31P{1H} NMR (CDCl3, 122 MHz,) δ: 142.81. MS (FAB+; m/z) 628 [M]+. Elem. Anal. Calcd. for C32H25ClNiO4P2: C, 61.04; H, 4.00. Found: C, 61.17; H, 4.08 %. HPLC-PDA purity: 97.62 %.
In Vitro Evaluation
Cell Lines Culture
The cytotoxicity of complexes 2 a–2 c was screened in vitro against six human cancer cell lines: HCT-15 (human colorectal adenocarcinoma), MCF-7 (human mammary adenocarcinoma), K562 (human chronic myelogenous leukemia), U251 (human glioblastoma), PC-3 (human prostatic adenocarcinoma), SKLU-1 (human lung adenocarcinoma), and a healthy cell line COS-7 (African green monkey kidney). All cell lines were supplied by National Cancer Institute (USA) and cultured in RPMI-1640 medium supplemented with 10 % fetal bovine serum, 2 mM L-glutamine, 10,000 units/ml penicillin G sodium, 10,000 μg/ml streptomycin sulfate and 25 μg/ml amphotericin B (Gibco) and 1 % non-essential amino acids (Gibco). They were maintained at 37 °C in humidified atmosphere with 5 % CO2. The viability of the cells used in the experiments exceeds 95 % as determined with trypan blue.
Cytotoxic Assay
The cytotoxicity was determined by the protein-binding dye sulforhodamine B (SRB) assay. The test is based on the ability of SRB to bind to protein components of cells that have been fixed to tissue culture plates by trichloroacetic acid (TCA), the amount of dye extracted from stained cells is directly proportional to the cell mass. The cells were removed from the culture flasks by treatment with trypsin, diluted with Dulbecco's Modified Eagle's Medium (DMEM) containing 10 % (v/v) of fetal bovine serum (FBS) and plated onto 96-well plates at a density 10,000 cell cm3 in a volume of 100 μL. The culture was incubated at 37 °C, 5 % CO2, and 90 % humidified air for 24 h. Subsequently, 100 μL of methanolic solution of each complex at 50 μM, and 100 μL of DMEM medium were added to each well. The cells were incubated at 37 °C, 5 % CO2 and 90 % humidified air for 48 h. The medium was removed, and the SRB assay was performed following the reported technique.27 After the incubation period, cells were fixed to the plastic substratum by addition of 50 μL of cold 50 % aqueous trichloroacetic acid. The plates were incubated at 4 °C for 1 h, washed with tap water, and air-dried. The trichloroacetic-acid-fixed cells were stained by the addition of 0.4 % SRB. Free SRB solution was removed by washing with 1 % aqueous acetic acid. The plates were then air-dried, and the bound dye was solubilized by the addition of 10 mM tris base buffer (100 μL). The plates were shaked for 10 min, and absorbance was determined at 515 nm using an ELISA plates reader (Bio-Tex Instruments).
Computational Details
Molecular Docking
The cytotoxic essays highlight the activity of compounds 1 b and 2 b towards the K-562 (chronic myelogenous leukemia) and MCF-7 (breast cancer) cell lines; in a search for potential biological targets for these cancer types, we found 14 proteins for leukemia among membrane receptors, tyrosine kinases, and cyclin-dependent kinases28-31 (PDBs: 3CS9, 5vkm, 5xy1, 6hm6, 6hrp, 1qpj, 1xz0, 2dq7, 4xjs, 1w98, 2w96, 3g33, 3nup, and 1fin),32-42 and 5 proteins for breast cancer among kinases, synthetases, and progesterone and estrogen receptors43-45 (PDBs: 2j6m, 3hy3, 4fa2, 4oar, 6chz).46-50 The biological targets were prepared in the software Maestro 10.3, assigning bonding orders, deleting water molecules and substrates, and preoptimizing the residues to a pH of 7.0. Gasteiger charges were then added to them in AutoDock Tools 1.5.6,51 preserving only polar hydrogens. The structures for the compounds 1b and 2 b were taken from the x-ray crystal results and electronic structure single-point calculations were performed for them in the Gaussian 16 computational package52 to approach the necessary charges via NBO method. The level of theory for the latter was DFT/M06-2X, using the 6–31++g(d,p) basis set for all non-metallic atoms and the LanL2DZ pseudopotential for the Ni centers, including the implicit solvation model SMD/H2O. Then, Gasteiger charges were added for the ligands in AutoDock Tools 1.5.6. The molecular docking simulations were carried out on AutoDock 4.2.6,51 where 100 molecular dockings were performed for each protein-ligand complex, making sure the grid boxes covered the whole active site of each target. The Lamarkian genetic algorithm was used with 25 M max. number of evaluations and 300 individuals. The results were analyzed to produce 3D representations of the binding site with Maestro 10.3 and 2D representations with LigPlot+.53, 54
2 Results
2.1 Synthesis of Para-Acetylated Functionalized Ni(II)-POCOP Pincer Complexes
para-acetylated pincer complexes were synthesized using the para-hydroxy Ni(II)-POCOP precursors, which were obtained following a procedure previously reported by our group.26 The first step of this procedure involves the reaction of 1,3,5-trihydroxybenzene with two equivalents of chlorophosphines ClPR2 (R=iPr, tBu, Ph) to generate the corresponding POCOP ligands [C6H3-5-OH-1,3-(OPR2)2]. Subsequently, a metalation reaction of the pincer ligand with an equivalent of NiCl2 ⋅ 6H2O in dry toluene was carried out to yield the corresponding para-hydroxy POCOP complexes with the formula [NiCl{C6H2-4-OH-2,6-(OPR2)2}] (R=iPr (1 a), tBu (1b), Ph (1c)).
With the pincer complexes 1 a–1 c in hand, the preparation of the para-acetoxy POCOP−Ni(II) complexes (2 a–c) was carried out using two synthetic strategies. Firstly, a direct acetylation of complexes 1 a and 1 b was conducted using acetyl chloride and potassium carbonate in acetone as a solvent, resulting in the para-acetoxy pincer complexes 2 a and 2 b with isolated yields of 72.2 % and 70.3 %, respectively (Scheme 2). On the other hand, to enhance the yield of the acetylation reaction for derivative 2 c, the reaction was performed in two steps. First, the phenolate moiety was generated by the deprotonation of the hydroxy group using sodium tetramethoxyborate in dry THF. In this step, the solution changed from a bright yellow color to dark red. Then, acetyl chloride was added to the reaction mixture, affording the acetylated product 2 c with a 59.2 % yield (Scheme 2).
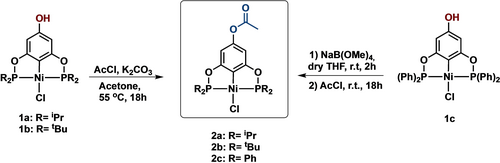
General procedure for the synthesis of complexes 2 a–2 c
The 1H-NMR spectra of Ni-POCOP complexes 2 a–c revealed C2v symmetry. The characteristic singlet assigned to the −COCH3 fragment in all complexes was observed in the range of 2.23–2.26 ppm. In the 13C{1H} spectra, the signal of the metalated carbon (C−Ni) appears as a triplet due to its coupling with two equivalent 31P nuclei at 121.82, 121.27, and 123.17 ppm for 2 a, 2 b, and 2 c, respectively. Meanwhile, the carbon of the carbonyl from the acetyl group was observed at approximately 169 ppm in all compounds. The 31P{1H} spectroscopy showed a single signal at 187.03, 189.56, and 142.81 ppm for 2 a, 2 b, and 2 c, respectively, confirming the symmetry of the pincer complexes. The mass spectra of 2 a–2 c provided additional information regarding their molecular structures. The molecular ions [M]+ of 2 a, 2 b and 2 c were presented at 492, 548 and 628 m/z, respectively. All these data agree with the proposed molecular structures.
In addition, the molecular structure of complex 2 b was elucidated through single crystal X-ray diffraction analysis (Figure 2). Suitable crystals for the study of 2 b were obtained by slowly diffusing hexane into a saturated ethyl acetate solution of the compound at room temperature. Compound 2 b crystallized in the centrosymmetric space group P-1, and the unit cell values are presented in Table 1.
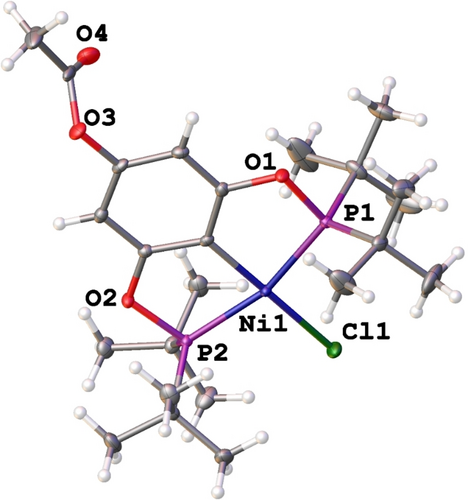
Molecular structure of (2 b), ellipsoids are represented at 50 % probability level.
Empirical formula |
C24 H41ClNiO4P2 |
|---|---|
Formula weight |
549.67 |
Temperature (K) |
100(2) |
Wavelength |
0.71073 Å |
Crystal system |
Triclinic |
Space group |
P-1 |
Unit cell dimensions |
a=8.0535(4) Å α=105.6900(10)° b=12.6701(7) Å β=96.6620(10)° c=14.7261(8) Å γ=101.3740(10)° |
Volume (Å3) |
1395.22(13) |
Z |
2 |
Densitycalcd (g/m3) |
1.308 |
Absorption coefficient (mm−1) |
0.932 |
F(0 0 0) |
584 |
Crystal size (mm3) |
0.225x0.131x0.109 |
Theta range for data collection |
2.570 to 25.679° |
Index ranges |
−9<=h<=9, −15<=k<=15, −17<=l<=17 |
Reflections collected |
22990 |
Independent reflections |
5256 [R(int)=0.0181] |
Completeness to theta |
99.7 % |
Absorption correction |
Semi-empirical from equivalents |
Max. and min. transmission |
0.7452 and 0.6950 |
Refinement method |
Full-matrix least-squares on F2 |
Data/restraints/parameters |
5256/0/302 |
Goodness-of-fit on F2 |
1.053 |
Final R indices [I>2sigma(I)]% |
R=0.0305, wRw=0.0796 |
R indices (all data)/ % |
R=0.0319, wRw=0.0806 |
Largest diff. peak and hole |
1.501 and −0.312 e.Å−3 |
The nickel center exhibited a slightly distorted square planar geometry, coordinated to the pincer POCOP ligand in a tridentate fashion via two phosphorus atoms (P1, P2) and a sigma C2−Ni, forming two five-membered metallacycles. The coordination sphere was completed with a chloride. Furthermore, an acetoxy fragment at position 4 of the main phenyl ring was observed. Selected bond lengths and angles are summarized in Table 2. Additionally, the acetoxy moiety is involved in the formation of a dimeric macrocycle with a C(20)-H(20)⋅⋅⋅O(4) distance of 2.558 Å and an angle of 164.31° [graph set R22(22)] (Figure 3a, Table 3). An interesting interaction was also observed between C14-H14 C and C5 (2.691 Å), forming a polymeric chain (Figure 3b, Table 3).
Ni1−C2 |
1.8826(17) Å, |
Ni1−P1 |
2.1918(5) Å, |
|---|---|---|---|
Ni1−P2 |
2.1828(5) Å, |
Ni1−Cl1 |
2.2013(5) Å, |
C2−Ni1−P2 |
81.74(6)° |
C2−Ni1−Cl1 |
179.69(6)° |
C2−Ni1−P1 |
82.27(6)° |
P2−Ni1−Cl1 |
97.968(19)° |
P2−Ni1−P1 |
163.84(2)° |
P1−Ni1−Cl1 |
98.028(19)° |
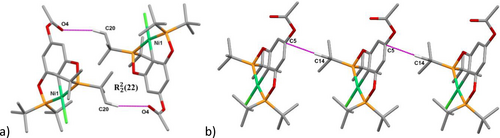
Principal interactions of the crystal packing for 2 b. a) A dimeric macrocycle [graph set (22)] and b) polymeric CH⋅⋅⋅C interaction. Hydrogens not involved in interactions are omitted for clarity.
D−H ⋅⋅⋅A |
D−H (Å) |
H ⋅⋅⋅A (Å) |
D ⋅⋅⋅A (Å) |
∡D−H ⋅⋅⋅A (°) |
|---|---|---|---|---|
C20−H20⋅⋅⋅O4(i) |
0.980 |
2.558 |
3.512 |
164.31 |
C14-H14 ⋅⋅⋅C5(ii) |
0.981 |
2.691 |
3.658 |
169.01 |
- Symmetry codes: (i) 1-x, 1-y,2-z. (ii) 1+x, y, z.
To analyse and visualize the closed intermolecular atomic interactions in the crystallographic packing of 2 b, a Hirshfeld surface analysis was performed using Crystal Explorer software.55, 56 The surface area was determined using the descriptor dnorm. Intensive red-hot spots on the surface, colored according to dnorm, are associated with interactions involving the ester moiety and the isopropyl group (Figure 4a). Additionally, a 2D fingerprint was plotted, depicted in Figure 4b, and the percentage of the individual contribution of each contact is shown in Figure 4c.
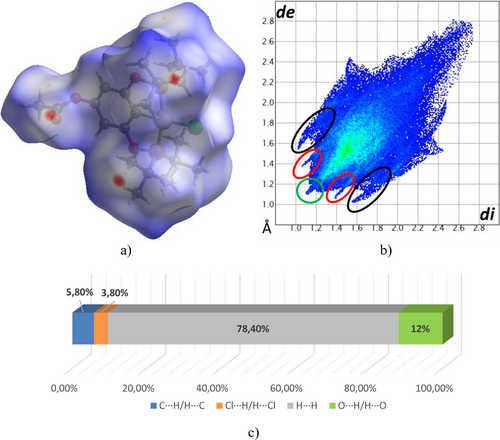
a) Hirshfeld surface mapped on dnorm, b) Fingerprint plot, and c) Percentages of the individual contribution of contacts in complex 2b.
The fingerprint shows three characteristic peaks, two of which are symmetrical peaks assigned to O⋅⋅⋅H/H⋅⋅⋅O (12 %) and C⋅⋅⋅H/H⋅⋅⋅C (5.8 %) contacts (red and black ellipsoids, respectively), and a central peak due to H/H (78.4 %) contacts (green circle). Also, in the decomposed plot of the individual contributions in fingerprints, halogen-hydrogen Cl⋅⋅⋅H/H⋅⋅⋅Cl (3.8 %) contacts were identified. The analysis of the relative percentage contributions of close contacts to the Hirshfeld surface in compound 2 b (Figure 4c.) suggests that intermolecular H⋅⋅⋅H contacts are the most abundant interactions in the compound.
2.2 Cytotoxic Evaluation
The in vitro cytotoxic activity of all complexes was evaluated against six cancer cell lines: glioblastoma (U-251), prostatic adenocarcinoma (PC-3), lymphoma of chronic myelogenous leukemia (K-562), colorectal adenocarcinoma (HCT-15), mammary adenocarcinoma (MCF-7), and lung adenocarcinoma (SKLU-1), along with a non-cancerous green monkey kidney (COS-7) cell line for comparative purposes. The effects of all non-functionalized Ni(II)-POCOP (1 a–c) and para-acetylated functionalized Ni(II)-POCOP pincer complexes on the growth of these six cancer cell lines are listed in Table 4. In general, the non-functionalized Ni(II)-POCOP (1 a–c) showed higher cytotoxic activity than the para-acetylated functionalized Ni(II)-POCOP pincer complexes in almost all screened cancer cell lines (except for MCF-7) including the healthy cell line COS-7. On the other hand, when analyzing the effect of substituents on the phosphine arm, it was observed that the role of these substituents is crucial. This fact was evidenced by the results of 1 a and 2 b, both containing -P(iPr)2 groups, being extremely active in all the evaluated cell lines, including the healthy cell line COS-7. In general, complexes with tBu and Ph on the phosphine exhibited less activity against cancer cell lines compared to those bearing iPr.
|
U-251 |
PC-3 |
K-562 |
HCT-15 |
MCF-7 |
SKLU-1 |
COS-7 |
|---|---|---|---|---|---|---|---|
1 a |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
1 b |
44.3 |
61.2 |
48.3 |
55.7 |
63.6 |
98.1 |
47.5 |
1 c |
100 |
94.1 |
81.7 |
81.5 |
26.8 |
100 |
79.3 |
2 a |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
2 b |
– |
– |
43.6 |
– |
52.7 |
15.4 |
1.3 |
2 c |
48.5 |
44.8 |
71.8 |
25.7 |
51.5 |
55.2 |
7.8 |
cisplatin |
92.3 |
42.9 |
66.4 |
38.3 |
56.2 |
85.1 |
66.3 |
On the other hand, the acetylated derivative 2 b exhibited no inhibition against U-251, PC-3, and HCT-15, while the inhibition rates for K-562 and MCF-7 were good, reaching up to 53 %. Complex 2 c showed remarkable activity against K-562 with an inhibition rate of 71.8 %, compared with the other cell lines. Importantly, complexes 2 b (1.3 %) and 2 c (7.8 %) showed lower activity against the healthy line COS-7 compared to their non-functionalized Ni(II)-POCOP counterparts 1 b (47.55) and 1 c (79.3). According to these results, the presence of an acetyl moiety on complexes 2 b and 2 c could offer a positive effect on cytotoxic activity, showing notable selectivity towards specific cancer lines along with very low inhibition against healthy cells. This fact being of utmost importance since new metallodrugs are sought to be both safer and more efficient.
Since compound 2 b was selective toward K-562 and MCF-7 and practically inactive in healthy cells (1.3 %), in contrast to cisplatin (66.3 %), its IC50 parameters on these two human cancer cell lines were determined. Compound 2 b exhibited good cytotoxicity against K-562 (7.32±0.60 μM) and MCF-7 (14.36±0.02 μM). These values are similar to those found for cisplatin (Table 5).
Compound |
K-562 (μM) |
MCF-7 (μM) |
|---|---|---|
2 b |
7.32±0.60 |
14.36±0.02 |
cisplatin |
8.6±0.9 |
5.5±0.5 |
2.3 Molecular Docking Simulations
The different binding modes of compounds 1 b and 2 b with the set of chosen proteins for K-562 and MCF-7 cell lines were explored. Our primary focus was on identifying the binding modes with the highest scores. The scoring values obtained from the docking simulations are presented in Table 6, clearly demonstrating that all the obtained scores fall within the range of −1.82 to −4.18. According to the results, the strongest interactions are to be expected for the cluster differentiation 1a protein (CD1 A, PDB: 1xz0), in the case of the K-562 cell line; and the progesterone receptor (PDB: 4oar), for the MCF-7 cell line. This fact applies to both compounds 1 b and 2 b.
K-562 |
||
|---|---|---|
Protein |
1 b |
2 b |
5vkm |
−2.67 |
−2.68 |
5xy1 |
−3.81 |
−2.68 |
6hm6 |
−2.85 |
−2.85 |
6hrp |
−2.41 |
−2.84 |
3cs9 |
−2.32 |
−2.33 |
1fin |
−2.72 |
−3.37 |
1w98 |
−3.28 |
−3.62 |
2w96 |
−2.80 |
−3.67 |
3g33 |
−3.33 |
−3.50 |
3nup |
−2.58 |
−2.53 |
1qpj |
−2.58 |
−3.48 |
1xz0[a] |
−4.18 |
−3.93 |
2dq7 |
−1.82 |
−2.99 |
4xjs |
−3.65 |
−3.32 |
MCF-7 |
||
Protein |
1 b |
2 b |
2j6m |
−2.42 |
−2.27 |
3hy3 |
−2.20 |
−2.73 |
4fa2 |
−3.26 |
−3.11 |
4oar[a] |
−3.69 |
−4.15 |
6chz |
−3.27 |
−3.96 |
- [a] Protein targets with the best scoring values for both compounds.
Given our keen interest in analyzing the interactions with these specific protein targets, the optimal binding poses obtained are represented in Figure 5. In all cases the compounds enter to the active site with their chloride and tert-butyl groups on the inside, leaving the ring substituted group oriented to the outside of the pockets. In both scenarios with protein CD1 A and the progesterone receptor, the compounds exhibit a predominant hydrophobic binding mode involving several aminoacids, which is attributed to the numerous interactions with their bulky tert-butyl fragments. In addition, both compounds present an extra hydrogen bonding when binding with protein CD1 A, ocurring between the aminoacid ASN 151 and the hydroxyl group for 1 b, and the acetoxy group in the case of 2 b. A detailed effect of the ring-group substitution from the hydroxyl in 1 b to the acetylated derivative 2 b cannot be further explored by these means, however, the docking simulations suggest that no big effect takes place in the binding, or, at least, not on these targets, as both bind to the exact same sites, with similar interactions and scoring values.
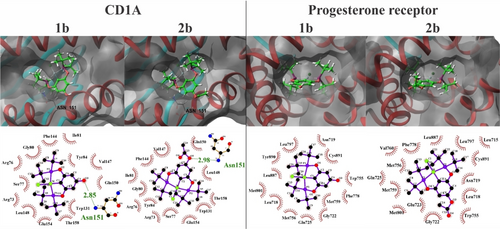
3D and 2D representations for the best interacting poses for compounds 1b and 2b towards CD1 A (PDB: 1xz0) and the progesterone receptor (PDB: 4oar). The compounds are presented in green ball & stick fashion, and the protein targets have their secondary structure highlighted in red (alpha helix), and light blue (beta sheets).
3 Conclusions
In summary, a series of acetylated POCOP−Ni(II) complexes were successfully synthesized and characterized. The molecular structure of complex 2 b has also been determined by single-crystal X-ray diffraction studies, confirming that the nickel center is in a square-planar coordination environment with the pincer ligand coordinated in a tridentate fashion, and the acetyl fragment positioned at the 4-position on the main aryl backbone of the pincer system. Additionally, the crystal packing is predominantly stabilized by acetoxy-isopropyl intermolecular interactions, facilitating the formation of two hydrogen bonds between pairs of molecules.
The in vitro cytotoxic studies of the para-acetylated functionalized Ni(II)-POCOP pincer complexes revealed that complex 2 b, which contains the tBu substituents in the phosphine moiety, exhibited significant growth inhibition of lymphoma of chronic myelogenous leukemia (K-562) and mammary adenocarcinoma (MCF-7), while being almost inactive against healthy cells (COS-7). In general, the presence of an acetyl moiety increased selectivity towards cancer cells. Interestingly, complex 2 b showed an IC50 value lower than that of cisplatin against K-562.
Regarding molecular docking, it was possible to identify that complex 2 b interacts with cluster differentiation protein 1a (CD1 A, PDB: 1xz0) as well as the progesterone receptor (PDB: 4oar) with score function values of −3.93 and −4.15, respectively. This result suggests that these could be the protein targets for the complex against the cancer cell lines K-562 and MFC-7.
Given the promising outcomes of this work, our future efforts will focus on conducting further mechanistic studies on the anticancer action of nickel complex 2 b. Our aim is to design and develop alternative Ni(II) complexes as anticancer agents.
Crystallography
Deposition Number CCDC 2345069 (for 2 b) (https://www.ccdc.cam.ac.uk/services/structures?id=doi:10.1002/cbdv.202400995) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe “http://www.ccdc.cam.ac.uk/structures” Access Structures service.
Author Contributions
The synthesis of the compounds was performed by Arturo Sánchez-Mora, Edwin Briñez, Alejandro Pico and J. Antonio Cruz-Navarro under David Morales-Morales supervision. Antonino Arenaza-Corona performed the supramolecular analyses. Nicolás Puentes-Díaz and Jorge Alí-Torres performed theoretical calculations and docking experiments. The research idea was proposed by David Morales-Morales, who together with Arturo Sánchez-Mora and J. Antonio Cruz-Navarro prepared all the figures and wrote the manuscript. David Morales-Morales, Lucero González-Sebastián and Viviana Reyes-Márquez reviewed the manuscript. All authors approved the final version of the manuscript. Funding was obtained and granted to David Morales-Morales.
Acknowledgments
We would like to thank M.Sc. Teresa Ramírez-Apan, M. Sc. Simon Hernandez-Ortega, Dr. Diego Martínez Otero, Dr. Francisco Javier Pérez Flores, Q. Eréndira García Ríos, M.Sc. Lucia del Carmen Márquez Alonso, M.Sc. Lucero Ríos Ruiz, M.Sc. Alejandra Núñez Pineda (CCIQS), Q. María de la Paz Orta Pérez and Ph.D. Nuria Esturau Escofet for technical assistance. Arturo S. would like to thank CONAHCyT (No. CVU: 848539) and Fundación Telmex-Telcel (Folio: 212009996) for a PhD scholarship. J.A.C.N (CVU: 824190) wants to acknowledge CONAHCyT for the postdoctoral fellowship provided (Estancias postdoctorales por México-2022 (III)). A.A.C. is grateful to CONAHCyT (CVU: 565984) for the postdoctoral fellowship (Estancias postdoctorales por México 2022). The financial support of this research by CONAHCYT (A1-S-033933) and DGAPA PAPIIT (IN223323) is gratefully acknowledged.
Conflict of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Open Research
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.