Progress in the Total Synthesis of Antitumor Tetrahydroisoquinoline Alkaloids
Yi Gao
Department Key Laboratory for Green Chemical Process of Ministry of Education, Wuhan Institute of Technology, Wuhan, 430205 China
Search for more papers by this authorNi Tu
Department Key Laboratory for Green Chemical Process of Ministry of Education, Wuhan Institute of Technology, Wuhan, 430205 China
Search for more papers by this authorXiaoting Liu
Department Key Laboratory for Green Chemical Process of Ministry of Education, Wuhan Institute of Technology, Wuhan, 430205 China
Search for more papers by this authorKangkang Lu
Department Key Laboratory for Green Chemical Process of Ministry of Education, Wuhan Institute of Technology, Wuhan, 430205 China
Search for more papers by this authorSiyu Chen
Department Key Laboratory for Green Chemical Process of Ministry of Education, Wuhan Institute of Technology, Wuhan, 430205 China
Search for more papers by this authorCorresponding Author
Ju Guo
Department Key Laboratory for Green Chemical Process of Ministry of Education, Wuhan Institute of Technology, Wuhan, 430205 China
Search for more papers by this authorYi Gao
Department Key Laboratory for Green Chemical Process of Ministry of Education, Wuhan Institute of Technology, Wuhan, 430205 China
Search for more papers by this authorNi Tu
Department Key Laboratory for Green Chemical Process of Ministry of Education, Wuhan Institute of Technology, Wuhan, 430205 China
Search for more papers by this authorXiaoting Liu
Department Key Laboratory for Green Chemical Process of Ministry of Education, Wuhan Institute of Technology, Wuhan, 430205 China
Search for more papers by this authorKangkang Lu
Department Key Laboratory for Green Chemical Process of Ministry of Education, Wuhan Institute of Technology, Wuhan, 430205 China
Search for more papers by this authorSiyu Chen
Department Key Laboratory for Green Chemical Process of Ministry of Education, Wuhan Institute of Technology, Wuhan, 430205 China
Search for more papers by this authorCorresponding Author
Ju Guo
Department Key Laboratory for Green Chemical Process of Ministry of Education, Wuhan Institute of Technology, Wuhan, 430205 China
Search for more papers by this authorAbstract
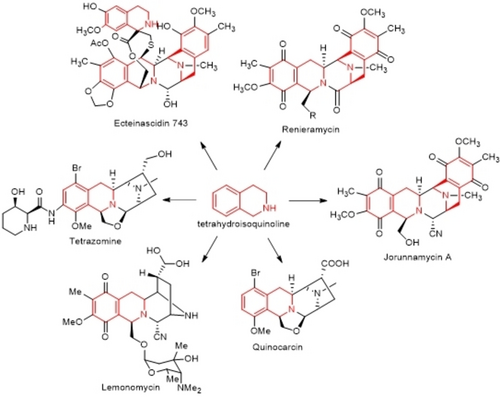
Among the tetrahydroisoquinoline(THIQ) of natural products, a family of THIQ alkaloids has the characteristics of similar biosynthetic pathway. Such THIQ alkaloids family mainly include Renieramycins, Ecteinasicdins, Tetrazaomine, Lemonomycin, etc. Most of these natural compounds have strong antitumor activities, and its family member Ecteinasicdins743 (ET-743, Trabectedin) has been marketed in the European Union and the United States for the treatment of advanced soft tissue tumors and ovarian cancer. Because of the excellent biological activity and complex chemical structure of this kind of THIQ products, it has aroused great interest of biologists and chemists, and many synthetic chemists have paid considerable efforts to their total synthesis over the past decade. Based on this, the recent advances in the total synthesis of such THIQ alkaloids are reviewed.
Graphical Abstract
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1J. D. Scott, R. M. Williams, ‘ChemInform Abstract: Chemistry and Biology of the Tetrahydroisoquinoline Antitumor Antibiotics’, Chem. Rev. 2002, 102, 1669–1730.
- 2K. Ishiguro, S. Sakiyama, K. Takahashi, T. Arai, ‘Mode of action of saframycin A, a novel heterocyclic quinone antibiotic. Inhibition of RNA synthesis in vivo and in vitro’, Biochemistry 1978, 17, 2545–2550.
- 3B. S. Davidson, ‘Renieramycin G, a new alkaloid from the sponge Xestospongia caycedoi’, Tetrahedron Lett. 1992, 33, 3721–3724.
- 4A. Fontana, P. Cavaliere, S. Wahidulla, C. G. Naik, G. Cimino, ‘A New Antitumor Isoquinoline Alkaloid from the Marine Nudibranch Jorunna funebris’, Tetrahedron 2000, 56, 7305–7308.
- 5G. Valoti, M. I. Nicoletti, A. Pellegrino, J. Jimeno, H. Hendriks, M. D′Incalci, G. Faircloth, R. Giavazzi, ‘Ecteinascidin-743, a new marine natural product with potent antitumor activity on human ovarian carcinoma xenografts’, Clin. Cancer Res. 1998, 4, 1977–1983.
- 6F. Tomita, K. Takahashi, K. I. Shimizu, ‘DC-52, a novel antitumor antibiotic. 1. Taxonomy, fermentation and biological activity’, J. Antibiot. 1983, 36, 463–467.
- 7F. Tomita, K. Takahashi, T. Tamaoki, ‘Quinocarcin, a novel antitumor antibiotic 3. mode of action’, J. Antibiot. 1984, 37, 1268–1272.
- 8K. Fujimoto, T. Oka, M. Morimoto, ‘Antitumor activity of a novel antitumor antibiotic, quinocarmycin citrate (KW2152)’, Cancer Res. 1987, 47, 1516–1522.
- 9K. Suzuki, T. Sato, M. Morioka, K. Nagai, K. Abe, H. Saito, T. Yamaguchi, ‘Tetrazomine, a new antibiotic produced by an actinomycete strain. Taxonomy, fermentation, isolation and characterization’, J. Antibiot. 1991, 44, 479–485.
- 10H. A. Whaley, E. L. Patterson, M. Dann, A. J. Shay, J. N. Porter, ‘Isolation and characterization of Lemonomycin, a new antibiotic’, Antimicrob. Agents Chemother. 1964, 8, 83–86.
- 11E. J. Martinez, E. J. Corey, T. Owa, ‘Antitumor activity- and gene expression-based profiling of ecteinascidin Et-743 and phthalascidin Pt 650’, Chem. Biol. 2001, 8, 1151–1160.
- 12H. Halim, P. Chunhacha, K. Suwanborirux, P. Chanvorachote, ‘Anticancer and Antimetastatic Activities of Renieramycin M, a Marine Tetrahydroisoquinoline Alkaloid, in Human Non-small Cell Lung Cancer Cells’, Anticancer Res. 2011, 31, 193–201.
- 13V. H. Le, M. Inai, R. M. Williams, T. Kan, ‘Ecteinascidins. A review of the chemistry, biology and clinical utility of potent tetrahydroisoquinoline antitumor antibiotics’, Nat. Prod. Rep. 2015, 32, 328–347.
- 14S. Inaba, M. Shimoyama, ‘Antitumor Activity of Quinocarmycin (KW2152) against Various Cultured Leukemia and Lymphoma Cell Lines in Vitro’, Cancer Res. 1988, 48, 6029–6032.
- 15J. R. Jett, N. Saijo, W. S. Hong, Y. Sasaki, H. Takahashi, H. Nakano, N. K. akagawa, M. Sakurai, K. Suemasu, M. I. Tesada, ‘The colony inhibition of a new chemotherapeutic agent (KW2152) against human lung cancer cell lines’, New Drugs. 1987, 5, 155–159.
- 16J. Plowman, D. J. Dykes, V. L. Narayanan, B. J. Abbott, H. Saito, T. Hirata, M. R. Grever, ‘Efficacy of the quinocarmycins KW2152 and DX-52-1 against human melanoma lines growing in culture and in mice’, Cancer Res. 1995, 55, 862–867.
- 17C. A. Bunnell, J. G. Supko, J. P. Eder, J. W. Clark, T. J. Lynch, D. W. Kufe, L. N. Shulman, ‘Phase I clinical trial of 7-cyanoquinocarcinol (DX-52–1) in adult patients with refractory solid malignancies’, Cancer Chemother. Pharmacol. 2001, 48, 347–355.
- 18R. M. Williams, M. E. Flanagan, T. N. Tippie, ‘O2-dependent cleavage of DNA by tetrazomine’, Biochemistry. 1994, 33, 4086–4092.
- 19T. F. Molinski, D. S. Dalisay, S. L. Lievens, J. P. Saludes, ‘Drug development from marine natural products’, Nat. Rev. Drug Discovery 2009, 8, 69–85.
- 20C. Cuevas, M. Pérez, M. J. Martín, J. L. Chicharro, R. C. Fernádez, M. Flores, A. Francesch, P. Gallego, M. Zarzuelo, F. Calle, J. García, C. Polanco, I. Rodríguez, I. Manzanares, ‘Synthesis of Ecteinascidin ET-743 and Phthalascidin Pt-650 from Cyanosafracin B’, Org. Lett. 2000, 2, 2545–2548.
- 21R. Menchaca, V. Martínez, A. Rodríguez, N. Rodríguez, M. Flores, P. Gallego, I. Manzanares, C. Cuevas, ‘Synthesis of Natural Ecteinascidins (ET-729, ET-745, ET-759B, ET-736, ET-637, ET-594) from Cyanosafracin B’, J. Org. Chem. 2003, 68, 23, 8859–8866.
- 22E. J. Corey, D. Y. Gin, R. S. Kania, ‘Enantioselective total synthesis of ecteinascidin 743’, J. Am. Chem. Soc. 1996, 118, 9202–9203.
- 23J. M. Eduardo, E. J. Corey, ‘Enantioselective synthesis of saframycin A and evaluation of antitumor activity relative to ecteinascidin/saframycin hybrids’, Org. Lett. 1999, 1, 75–78.
- 24J. C. Chen, X. C. Chen, B. C. Michele, J. P. Zhu, ‘Total synthesis of ecteinascidin 743’, J. Am. Chem. Soc. 2006, 128, 87–89.
- 25X. Chen, J. Zhu, ‘Total Synthesis of the Marine Natural Product (−)-Cribrostatin 4 (Renieramycin H)’, Angew. Chem., Int. Ed. 2007, 21, 4306–4309.
- 26W. Jonathan, Y. Y. Chen, R. M. Williams, ‘Asymmetric Total Syntheses of (−)-Jorumycin, (−)-Renieramycin G, 3-epi-Jorumycin, and 3-epi-Renieramycin G’, J. Am. Chem. Soc. 2005, 127, 12684–12690.
- 27L. H. S. Smith, T. T. Nguyen, H. F. Sneddon, D. J. Procter, ‘Synthesis of the ABH rings of ecteinascidin 597 using a connective Pummerer-type cyclisation’, Chem. Commun. 2011, 47, 10821–10823.
- 28S. H. Xu, G. Wang, J. J. Zhu, C. Shen, Z. Z. Yang, J. Yu, Z. Li, T. H. Lin, X. Sun, F. L. Zhang, ‘A Concise and Practical Semisynthesis of Ecteinascidin 743 and (−)-Jorumycin’, Eur. J. Org. Chem. 2017, 5, 975–983.
- 29J. D. Scott, R. M. Williams, ‘Total Synthesis of (−)-Tetrazomine and Determination of Its Stereochemistry’, Angew. Chem., Int. Ed. 2001, 18, 1511–1513.
10.1002/1521-3757(20010417)113:8<1511::AID-ANGE1511>3.0.CO;2-A Google Scholar
- 30J. D. Scott, R. M. Williams, ‘Total Synthesis of (−)-Tetrazomine. Determination of the Stereochemistry of Tetrazomine and the Synthesis and Biological Activity of Tetrazomine Analogs’, J. Am. Chem. Soc. 2002, 124, 2951–2956.
- 31T. Fukuyama, J. Nunes, ‘Stereocontrolled total synthesis of (.+−.)-quinocarcin’, J. Am. Chem. Soc. 1988, 110, 5196–5198.
- 32K. Allan, B. M. Stoltz, ‘A Concise Total Synthesis of (−)-Quinocarcin via Aryne Annulation’, J. Am. Chem. Soc. 2008, 130, 17270–17271.
- 33R. W. Eric, N. Aurapat, K. Max, L. Guillaume, M. P. Gerit, S. J. M. Martina, C. Dylan, D. G. Christopher, M. T. Pamela, K. H. Christopher, N. Kenji, G. Emil, U. G. Christian, M. A. Kevin, C. V. Scott, J. S. Dennis, M. S. Brian, ‘Concise total syntheses of (−)-jorunnamycin A and (−)-jorumycin enabled by asymmetric catalysis’, Science. 2019, 363, 270–275.
- 34M. Yokoya, R. Toyoshima, T. Suzuki, V. H. Le, R. M. Williams, S. Naoki, ‘Stereoselective Total Synthesis of (−)-Renieramycin T’, J. Org. Chem. 2016, 81, 4039–4047.
- 35Y. Zheng, X. D. Li, P. Z. Sheng, H. D. Yang, K. Wei, Y. R. Yang, ‘Asymmetric Total Syntheses of (−)-Fennebricin A, (−)-Renieramycin J, (−)-Renieramycin G, (−)-Renieramycin M, and (−)- Jorunnamycin A via C−H Activation’, Org. Lett. 2020, 22, 4489–4493.
- 36S. L. Fang, M. X. Jiang, S. Zhang, Y. J. Wu, B. F. Shi, ‘Scalable Formal Synthesis of (−)-Quinocarcin’, Org. Lett. 2019, 21, 4609–4613.
- 37Y. Wang, Y. F. Tang, Z. Z. Liu, S. Z. Chern, X. T. Liang, ‘Synthetic Progress in Saframycins and Ecteinascidins’, Chin. J. Org. Chem. 2005, 25, 42–52.
- 38X. W. Liao, W. F. Dong, W. Liu, S. Z. Chen, Z. Z. Liu, ‘Synthetic Progress of the Tetrahydroisoquinoline Antitumor Alkaloids’, Chin. J. Org. Chem. 2010, 30, 317–329.
- 39Y. T. Song, L. L. Hu, R. J. Chen, X. C. Chen, ‘Research Progress in Synthesis of Renieramycin-Type Alkaloids’, Chin. J. Org. Chem. 2015, 35, 1627–1640.
- 40K. L. Rinehart, T. G. Holt, N. L. Fregeau, J. G. Stroh, P. A. Kieffer, F. Sun, L. H. Li, D. G. Martin, ‘Ecteinascidins 729, 743, 745, 759 A, 759B, and 770: potent antitumor agents from the Caribbean tunicate Ecteinascidia turbinata’, J. Org. Chem. 1990, 55, 4512–4515.
- 41A. Endo, A. Yanagisawa, M. Abe, S. Tohma, T. Kan, T. Fukuyama, ‘Total Synthesis of Ecteinascidin 743’, J. Am. Chem. Soc. 2002, 124, 6552–6554.
- 42F. Kawagishi, T. Toma, T. Inui, S. Yokoshima, T. Fukuyama, ‘Total Synthesis of Ecteinascidin 743’, J. Am. Chem. Soc. 2013, 135, 13684–13687.
- 43S. P. Zheng, C. Chen, T. Furuuchi, B. J. D. Wright, B. Zhou, J. Gao, S. J. Danishefsky, ‘Stereospecific Formal Total Synthesis of Ecteinascidin 743’, Angew. Chem. Int. Ed. 2006, 11, 1786–1791.
10.1002/ange.200503983 Google Scholar
- 44D. Fishlock, R. M. Williams, ‘Synthetic Studies on Et-743. Assembly of the Pentacyclic Core and a Formal Total Synthesis’, J. Org. Chem. 2008, 73, 9594–9600.
- 45W. He, Z. Zhang, D. Ma, ‘A Scalable Total Synthesis of the Antitumor Agents Et-743 and Lurbinectedin’, Angew. Chem. Int. Ed. 2019, 58, 3972–3975.
- 46R. Chen, D. Zhu, Z. Hu, Z. Zheng, X. Chen, ‘A new approach to the synthesis of l-3-hydroxy-4-methoxy-5-methyl-phenylalanine derivatives from l-tyrosine’, Tetrahedron: Asymmetry 2010, 21, 39–42.
10.1016/j.tetasy.2009.12.024 Google Scholar
- 47J. Jia, R. Chen, Y. Jia, H. Gu, Q. Zhou, X. C. Chen, ‘Convergent Formal Synthesis of Ecteinascidin 743’, J. Org. Chem. 2019, 84, 13696–13706.
- 48X. Chen, J. Chen, M. D. Paolis, J. Zhu, ‘Synthetic Studies toward Ecteinascidin 743’, J. Org. Chem. 2005, 70, 4397–4408.
- 49F. Guibe, Y. S. M‘Leux, ‘The allyloxycarbonyl group for alcohol protection: quantitative removal or transformation into allyl protecting group via π-allyl complexes of palladium’, Tetrahedron Lett. 1981, 22, 3591–3594.
- 50J. Lane, Y. Chen, R. M. Williams, ‘Asymmetric Total Syntheses of (−)-Jorumycin, (−)-Renieramycin G, 3-epi-Jorumycin, and 3-epi-Renieramycin G’, J. Am. Chem. Soc. 2005, 127, 12684–12690.
- 51Y. C. Wu, J. Zhu, ‘Asymmetric Total Syntheses of (−)-Renieramycin M and G and (−)-Jorumycin Using Aziridine as a Lynchpin’, Org. Lett. 2009, 11, 5558–5561.
- 52W. Liu, X. Liao, W. F. Dong, Z. Yan, N. Wang, Z. Z. Liu, ‘Total synthesis and cytotoxicity of (−)-jorumycin and its analogs’, Tetrahedron. 2012, 68, 2759–2764.
- 53R. Chen, H. Liu, X. Chen, ‘Asymmetric Total Synthesis of (−)-Jorunnamycins A and C and (−)-Jorumycin from l-Tyrosine’, J. Nat. Prod. 2013, 76, 1789–1795.
- 54Y. Zheng, X. D. Li, P. Z. Sheng, H. D. Yang, K. Wei, Y. R. Yang, ‘Asymmetric Total Syntheses of (−)-Fennebricin A, (−)-Renieramycin J, (−)-Renieramycin G, (−)-Renieramycin M, and (−)- Jorunnamycin A via C−H Activation’, Org. Lett. 2020, 22, 4489–4493.
- 55Y. C. Wu, G. Bernadat, G. Masson, C. Couturier, T. Schlama, J. Zhu, ‘Synthetic Studies on (−)-Lemonomycin: An Efficient Asymmetric Synthesis of Lemonomycinone Amide’, J. Org. Chem. 2009, 74, 2046–2052.
- 56J. He, S. Li, Y. Deng, H. Fu, B. N. Laforteza, J. E. Spangler, A. Homs, J. Q. Yu, ‘Ligand-Controlled C(sp3)-H Arylation and Olefination in Synthesis of Unnatural Chiral α-Amino Acids’, Science. 2014, 343, 1216–1220.
- 57B. Hartmann, A. M. Kanazawa, J. P. Depres, A. E. Greene, ‘Improved preparation of angelate esters’, Tetrahedron Lett. 1991, 32, 5077–5080.
- 58L. C. Campeau, D. J. Schipper, K. Fagnou, ‘Site-Selective sp2 and Benzylic sp3 Palladium-Catalyzed Direct Arylation’, J. Am. Chem. Soc. 2008, 130, 3266–3267.
- 59R. Dorta, D. Broggini, R. Stoop, H. Rüegger, F. Spindler, A. Togni, ‘Chiral Xyliphos Complexes for the Catalytic Imine Hydrogenation Leading to the Metolachlor Herbicide: Isolation of Catalyst-Substrate Adducts’, Chem. Eur. J. 2004, 10, 267–278.
- 60C. B. Lavery, N. L. Rotta-Loria, R. McDonald, M. Stradiotto, ‘Pd2dba3/Bippyphos: A Robust Catalyst System for the Hydroxylation of Aryl Halides with Broad Substrate Scope’, Adv. Synth. Catal. 2013, 355, 981–987.
- 61N. C. Bruno, M. T. Tudge, S. L. Buchwald, ‘Design and preparation of new palladium precatalysts for C−C and C−N cross-coupling reactions’, Chem. Sci. 2013. 4, 916–920.
- 62V. L. Ponzo, T. S. Kaufman, ‘Synthesis of the tetrahydroisoquinoline unit in the AB ring system of the novel antitumor-antibiotic tetrazomine’, J. Chem. Soc.-Perkin Trans. 1997, 1, 3131–3134.
10.1039/a706070j Google Scholar
- 63P. Wipf, C. R. Hopkins, ‘Enantioselective Synthesis of the AB-Ring System of the Antitumor Antibiotic Tetrazomine’, J. Org. Chem. 2001, 66, 3133–3139.
- 64W. Y. Qi, S. L. Fang, X. T. Xu, K. Zhang, B. F. Shi, ‘Asymmetric formal synthesis of (−)-tetrazomine’, Org. Chem. Front. 2021, 8, 1802–1807.
- 65L. Li, L. Shi, K. Wei, Y. R. Yang, ‘Asymmetric Total Synthesis of (+)-Quinocarcinamide’, Org. Lett. 2021, 23, 7972–7975.
- 66H. Giamarellou, ‘Treatment options for multidrug-resistant bacteria’, Expert Rev. Anti-Infect. Ther. 2006, 4, 601–618.
- 67P. Siengalewicz, U. Rinner, J. Mulzer, ‘Recent progress in the total synthesis of naphthyridinomycin and lemonomycin tetrahydro-isoquinoline antitumor antibiotics (TAAs)’, Chem. Soc. Rev. 2008, 37, 2676–2690.
- 68A. Yoshida, M. Akaiwa, T. Asakawa, Y. Hamashima, S. Yokoshima, T. Fukuyama, T. Kan, ‘Total Synthesis of (−)-Lemonomycin’, Chem.-Eur. J. 2012, 18, 11192–11195.
- 69J. Guo, W. F. Dong, W. Liu, Z. Yan, N. Wang, Z. Z. Liu, ‘Synthesis and cytotoxicity of 3-arylacrylic amide derivatives of the simplified saframycin-ecteinascidin skeleton prepared from l-dopa’, Eur. J. Med. Chem. 2013, 62, 670–676.
- 70J. Guo, Y. Yang, N. Wang, Z. Z. Liu, ‘Synthesis and cytotoxicity screening of derivatives of the simplified ecteinascidin pentacyclic skeleton as anticancer agents’, Tetrahedron Lett. 2018, 33, 3202–3205.