Anticholinesterase Compounds from Endemic Prangos uechtritzii
Gokay Albayrak
Department of Pharmaceutical Botany, Faculty of Pharmacy, İzmir Katip Çelebi University, 35620 İzmir, Turkey
Department of Pharmaceutical Botany, Faculty of Pharmacy, Ege University, 35040 İzmir, Turkey
Search for more papers by this authorSerdar Demir
Department of Pharmaceutical Botany, Faculty of Pharmacy, Ege University, 35040 İzmir, Turkey
Search for more papers by this authorHalil Koyu
Department of Pharmaceutical Botany, Faculty of Pharmacy, İzmir Katip Çelebi University, 35620 İzmir, Turkey
Search for more papers by this authorCorresponding Author
Sura Baykan
Department of Pharmaceutical Botany, Faculty of Pharmacy, Ege University, 35040 İzmir, Turkey
Search for more papers by this authorGokay Albayrak
Department of Pharmaceutical Botany, Faculty of Pharmacy, İzmir Katip Çelebi University, 35620 İzmir, Turkey
Department of Pharmaceutical Botany, Faculty of Pharmacy, Ege University, 35040 İzmir, Turkey
Search for more papers by this authorSerdar Demir
Department of Pharmaceutical Botany, Faculty of Pharmacy, Ege University, 35040 İzmir, Turkey
Search for more papers by this authorHalil Koyu
Department of Pharmaceutical Botany, Faculty of Pharmacy, İzmir Katip Çelebi University, 35620 İzmir, Turkey
Search for more papers by this authorCorresponding Author
Sura Baykan
Department of Pharmaceutical Botany, Faculty of Pharmacy, Ege University, 35040 İzmir, Turkey
Search for more papers by this authorAbstract
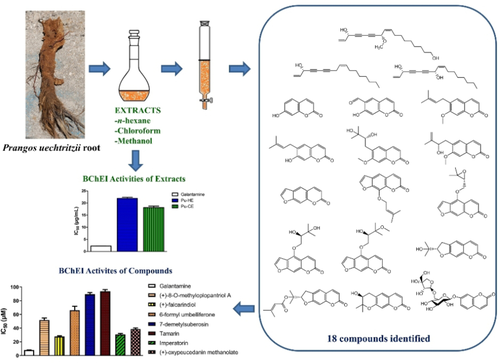
In this study, the anticholinesterase effects of the extracts and isolated compounds from the roots of endemic Prangos uechtritzii Boiss & Hausskn (Apiaceae) are reported. A novel polyacetylenic compound; (+)-8-O-methyloplopantriol A along with two known polyacetylenes; (−)-panaxynol, (+)-falcarindiol and fifteen known coumarin derivatives; umbelliferone, 6-formylumbelliferone, suberosin, 7-demethylsuberosin, (+)-ulopterol, tamarin, psoralen, imperatorin, (+)-oxypeucedanin, (+)-oxypeucedanin hydrate, (+)-oxypeucedanin methanolate, (+)-marmesin, (−)-prantschimgin, (+)-decursinol, and (−)-adicardin were isolated from the hexane (Pu-HE), chloroform (Pu-CE), and methanol (Pu-ME) extracts of P. uechtritzii roots. (−)-Panaxynol, (+)-falcarindiol, 6-formylumbelliferone, (+)-decursinol, and (−)-adicardin were obtained from the genus Prangos for the first time. (+)-8-O-Methyloplopantriol A inhibited both AChE (IC50=194.5±5.8 μM) and BChE (IC50=51.9±2.96 μM) enzymes. (+)-Falcarindiol, 6-formylumbelliferone, 7-demethylsuberosin, tamarin, and imperatorin also exhibited BChE-specific inhibitory activities (IC50=27.88-93.86 μM). (+)-Falcarindiol (IC50=27.88±0.91 μM) and imperatorin (IC50=30.89±1.40 μM) as the most active components could be led compounds to develop new BChE inhibitors with further research against Alzheimer's disease.
Graphical Abstract
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv202200557-sup-0001-misc_information.pdf642.4 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1E. McDade, R. J. Bateman, ‘Stop Alzheimer's before it starts’, Nature 2017, 547, 153–155.
- 2B. Sever, C. Turkes, M. D. Altintop, Y. Demir, S. Beydemir, ‘Thiazolyl-pyrazoline derivatives: In vitro and in silico evaluation as potential acetylcholinesterase and carbonic anhydrase inhibitors’, Int. J. Biol. Macromol. 2020, 163, 1970–1988.
- 3B. Sever, C. Turkes, M. D. Altintop, Y. Demir, G. Akalin-Ciftci, S. Beydemir, ‘Novel metabolic enzyme inhibitors designed through the molecular hybridization of thiazole and pyrazoline scaffolds’, Arch. Pharm. 2021, 354, e2100294.
- 4S. Askin, H. Tahtaci, C. Turkes, Y. Demir, A. Ece, G. Akalin-Ciftci, S. Beydemir, ‘Design, synthesis, characterization, in vitro and in silico evaluation of novel imidazo[2,1-b][1,3,4]thiadiazoles as highly potent acetylcholinesterase and non-classical carbonic anhydrase inhibitors’, Bioorg. Chem. 2021, 113, 105009.
- 5K. G. Yiannopoulou, S. G. Papageorgiou, ‘Current and future treatments for Alzheimer's disease’, Ther. Adv. Neurol. Disord. 2013, 6, 19–33.
- 6U. Yasar, I. Gonul, C. Turkes, Y. Demir, S. Beydemir, ‘Transition-Metal Complexes of Bidentate Schiff-Base Ligands: In vitro and in silico Evaluation as Non-Classical Carbonic Anhydrase and Potential Acetylcholinesterase Inhibitors’, ChemistrySelect 2021, 6, 7278–7284.
- 7W. V. Graham, A. Bonito-Oliva, T. P. Sakmar, ‘Update on Alzheimer's disease therapy and prevention strategies’, Annu. Rev. Med. 2017, 68, 413–430.
- 8N. H. Greig, D. K. Lahiri, K. Sambamurti, ‘Butyrylcholinesterase: An important new target in Alzheimer's disease therapy’, Int. Psychogeriatr. 2002, 14, 77–91.
- 9B. R. Pinho, F. Ferreres, P. Valentão, P. B. Andrade, ‘Nature as a source of metabolites with cholinesterase-inhibitory activity: An approach to Alzheimer's disease treatment’, J. Pharm. Pharmacol. 2013, 65, 1681–1700.
- 10M. Heinrich, H. L. Teoh, ‘Galanthamine from snowdrop - The development of a modern drug against Alzheimer's disease from local Caucasian knowledge’, J. Ethnopharmacol. 2004, 92, 147–162.
- 11D. J. Newman, G. M. Cragg, ‘Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019’, J. Nat. Prod. 2020, 83, 770–803.
- 12A. Murray, M. Faraoni, M. Castro, N. Alza, N. Cavallaro, ‘Natural AChE Inhibitors from plants and their contribution to Alzheimer's disease therapy’, Curr. Neuropharmacol. 2013, 11, 388–413.
- 13G. Albayrak, S. Demir, F. A. Kose, S. Baykan, ‘New coumarin glycosides from endemic Prangos heyniae H. Duman & M. F. Watson’, Nat. Prod. Res. 2021, 1–13.
- 14G. Bulut, E. Tuzlacı, A. Doğan, I. Şenkardes, ‘An ethnopharmacological review on the Turkish Apiaceae species’, J. Fac. Pharm. Istanbul Univ. 2014, 44, 163–179.
- 15J. Mottaghipisheh, T. Kiss, B. Tóth, D. Csupor, ‘The Prangos genus: a comprehensive review on traditional use, phytochemistry, and pharmacological activities’, Phytochem. Rev. 2020, 19, 1449–1470.
- 16G. Zengin, M. F. Mahomoodally, E. Yıldıztugay, S. Jugreet, S. U. Khan, S. Dall'Acqua, A. Mollica, A. Bouyahya, D. Montesano, ‘Chemical composition, biological activities and in silico analysis of essential oils of three endemic Prangos species from Turkey’, Molecules 2022, 27, 1676, 1–14.
- 17L. G. De Souza, O. B. M. N. Renn, J. D. Figueroa-Villar, ‘Coumarins as cholinesterase inhibitors: A review’, Chem.-Biol. Interact. 2016, 254, 11–23.
- 18C. Zidorn, K. Jöhrer, M. Ganzera, B. Schubert, E. M. Sigmund, J. Mader, R. Greil, E. P. Ellmerer, H. Stuppner, ‘Polyacetylenes from the Apiaceae vegetables carrot, celery, fennel, parsley, and parsnip and their cytotoxic activities’, J. Agric. Food Chem. 2005, 53, 2518–2523.
- 19S. Dall'Acqua, F. Maggi, P. Minesso, M. Salvagno, F. Papa, S. Vittori, G. Innocenti, ‘Identification of non-alkaloid acetylcholinesterase inhibitors from Ferulago campestris (Besser) Grecescu (Apiaceae)’, Fitoterapia 2010, 81, 1208–1212.
- 20K. Murata, D. Iida, Y. Ueno, K. Samukawa, T. Ishizaka, T. Kotake, H. Matsuda, ‘Novel polyacetylene derivatives and their inhibitory activities on acetylcholinesterase obtained from Panax ginseng roots’, J. Nat. Med. 2017, 71, 114–122.
- 21A. Guner, in ‘Türkiye bitkileri listesi:(damarlı bitkiler), ‘ Ed. A. Guner, Nezahat Gökyiǧit Botanik Bahçesi Yayınları, İstanbul, 2012, p. 75–76 (Turkish).
- 22T. Fujioka, K. Furumi, H. Fujii, H. Okabe, K. Mihashi, Y. Nakano, H. Matsunaga, M. Katano, M. Mori, ‘Antiproliferative constituents from Umbelliferae plants. V. A new furanocoumarin and falcarindiol furanocoumarin ethers from the root of Angelica japonica’, Chem. Pharm. Bull. 1999, 47, 96–100.
- 23R. Singh, B. Singh, S. Singh, N. Kumar, S. Kumar, S. Arora, ‘Umbelliferone - An antioxidant isolated from Acacia nilotica (L.) Willd. Ex. Del’, Food Chem. 2010, 120, 825–830.
- 24C. Ito, M. Matsuoka, T. Mizuno, K. Sato, Y. Kimura, M. Ju-Ichi, M. Inoue, I. Kajiura, M. Omura, H. Furukawa, ‘New coumarins from some Citrus plants’, Chem. Pharm. Bull. 1988, 36, 3805–3810.
- 25T. Dikpinar, S. Süzgeç-Selçuk, B. Ö. Çelik, E. A. Uruşak, ‘Antimicrobial activity of rhizomes of Ferulago trachycarpa Boiss. and bioguided isolation of active coumarin constituents’, Ind. Crops Prod. 2018, 123, 762–767.
- 26R. B. Filho, A. F. Magalhxes, R. Gottlieb, ‘Coumarins from Brosimum rubescens’, Phytochemistry 1972, 11, 3307–3310.
- 27A. G. Gonzalez, R. E. Reyes, M. R. Espino, ‘Two new coumarins from Ruta pinnata’, Phytochemistry 1977, 16, 2033–2035.
- 28C. Chunyan, S. Bo, L. Ping, L. Jingmei, Y. Ito, ‘Isolation and purification of psoralen and bergapten from Ficus carica L. leaves by high-speed countercurrent chromatography’, J. Liq. Chromatogr. Relat. Technol. 2009, 32, 136–143.
- 29K. Ghosh, ‘A furocoumarin, Imperatorin isolated from Urena lobata L. (Malvaceae)’, Molbank 2004, 2004, M382.
10.3390/M382 Google Scholar
- 30O. Gokay, D. Kuhner, M. Los, F. Gotz, U. Bertsche, K. Albert, ‘An efficient approach for the isolation, identification and evaluation of antimicrobial plant components on an analytical scale, demonstrated by the example of Radix Imperatoriae’, Anal. Bioanal. Chem. 2010, 398, 2039–2047.
- 31J. Hagemeier, O. Batz, J. Schmidt, V. Wray, K. Hahlbrock, D. Strack, ‘Accumulation of phthalides in elicitor-treated cell suspension cultures of Petroselinum crispum’, Phytochemistry 1999, 51, 629–635.
- 32S. N. Chang, I. Khan, D. K. Dey, K. H. Cho, B. S. Hwang, K. B. Bae, S. C. Kang, J. G. Park, ‘Decursinol angelate ameliorates 12-O-tetradecanoyl phorbol-13-acetate (TPA)-induced NF-κB activation on mice ears by inhibiting exaggerated inflammatory cell infiltration, oxidative stress and pro-inflammatory cytokine production’, Food Chem. Toxicol. 2019, 132, 110699.
- 33B. J. Nvau, B. Sami, O. S. Ajibade, I. A. Gray, J. O. Igoli, ‘Adicardin and other coumarins from Breonadia salicina (Vahl) Hepper’, Trop. J. Nat. Prod. Res. 2019, 3, 298–301.
- 34W. H. Huang, Q. W. Zhang, C. Z. Wang, C. S. Yuan, S. P. Li, ‘Isolation and identification of two new polyynes from a North American ethnic medicinal plant-Oplopanax horridus (Smith) Miq’, Mol. 2010, 15, 1089–1096.
- 35M. Loizzo, R. Tundis, F. Menichini, F. Menichini, ‘Natural products and their derivatives as cholinesterase inhibitors in the treatment of neurodegenerative disorders: An update’, Curr. Med. Chem. 2008, 15, 1209–1228.
- 36G. Zengin, K. I. Sinan, G. Ak, M. F. Mahomoodally, M. Y. Paksoy, C. Picot-Allain, J. Glamocilja, M. Sokovic, J. Jekő, Z. Cziáky, M. J. Rodrigues, C. G. Pereira, L. Custodio, ‘Chemical profile, antioxidant, antimicrobial, enzyme inhibitory, and cytotoxicity of seven Apiaceae species from Turkey: A comparative study’, Ind. Crops Prod. 2020, 153, 112572.
- 37M. Bruno, V. Ilardi, G. Lupidi, L. Quassinti, M. Bramucci, D. Fiorini, A. Venditti, F. Maggi, ‘Composition and biological activities of the essential oil from a Sicilian accession of Prangos ferulacea (L.) Lindl’, Nat. Prod. Res. 2021, 35, 733–743.
- 38M. B. Bahadori, G. Zengin, S. Bahadori, F. Maggi, L. Dinparast, ‘Chemical composition of essential oil, antioxidant, antidiabetic, anti-obesity, and neuroprotective properties of Prangos gaubae’, Nat. Prod. Commun. 2017, 12, 1945–1948.
- 39M. Abbas-Mohammadi, M. M. Farimani, P. Salehi, S. Nejad Ebrahimi, A. Sonboli, C. Kelso, D. Skropeta, ‘Acetylcholinesterase-inhibitory activity of Iranian plants: Combined HPLC/bioassay-guided fractionation, molecular networking and docking strategies for the dereplication of active compounds’, J. Pharm. Biomed. Anal. 2018, 158, 471–479.
- 40M. Y. Ali, S. H. Seong, M. R. Reddy, S. Y. Seo, J. S. Choi, H. A. Jung, ‘Kinetics and molecular docking studies of 6-formyl umbelliferone isolated from Angelica decursiva as an inhibitor of cholinesterase and BACE1’, Molecules 2017, 22, 1–13.
- 41Y. Kwon, H. P. Kim, M. J. Kim, W. Chun, ‘Acetylcholinesterase inhibitors from Angelica polymorpha Stem’, Nat. Prod. Sci. 2017, 23, 97–102.
- 42S. Y. Kang, K. Y. Lee, S. H. Sung, M. J. Park, Y. C. Kim, ‘Coumarins isolated from Angelica gigas inhibit acetylcholinesterase: Structure-activity relationships’, J. Nat. Prod. 2001, 64, 683–685.
- 43S. Karakaya, M. Koca, O. Sytar, B. Dursunoglu, H. Ozbek, H. Duman, Z. Guvenalp, C. S. Kilic, ‘Antioxidant and anticholinesterase potential of Ferulago cassia with farther bio-guided isolation of active coumarin constituents’, South. African. J. Bot. 2019, 121, 536–542.
- 44F. Golfakhrabadi, M. R. Shams Ardakani, S. Saeidnia, T. Akbarzadeh, F. Yousefbeyk, H. Jamalifar, M. Khanavi, ‘In vitro antimicrobial and acetylcholinesterase inhibitory activities of coumarins from Ferulago carduchorum’, Med. Chem. Res. 2016, 25, 1623–1629.
- 45Y. Tada, Y. Shikishima, Y. Takaishi, H. Shibata, T. Higuti, G. Honda, ‘Coumarins and γ-pyrone derivatives from Prangos pabularia: antibacterial activity and inhibition of cytokine release’, Phytochemistry 2002, 59, 649–654.
- 46K. Skalicka-Woźniak, I. E. Orhan, G. A. Cordell, S. M. Nabavi, B. Budzyńska, ‘Implication of coumarins towards central nervous system disorders’, Pharmacol. Res. 2016; 103, 188–203.
- 47S. Granica, A. K. Kiss, M. Jarończyk, J. K. Maurin, A. P. Mazurek, Z. Czarnocki, ‘Synthesis of imperatorin analogs and their evaluation as acetylcholinesterase and butyrylcholinesterase inhibitors’, Arch. Pharm. (Weinheim) 2013, 346, 775–782.
- 48N. Wszelaki, K. Paradowska, M. K. Jamróz, S. Granica, A. K. Kiss, ‘Bioactivity-guided fractionation for the butyrylcholinesterase inhibitory activity of furanocoumarins from Angelica archangelica L. roots and fruits’, J. Agric. Food Chem. 2011, 59, 9186–9193.
- 49Y. K. So, C. K. Young, ‘Neuroprotective coumarins from the root of Angelica gigas: Structure-activity relationships’, Arch. Pharmacal Res. 2007, 30, 1368–1373.
- 50C. A. Lipinski, F. Lombardo, B. W. Dominy, P. J. Feeney, ‘Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings’, Adv Drug Deliv Rev 2001, 46, 3–26.
- 51W. J. Egan, K. M. Merz Jr, J. J. Baldwin, ‘Prediction of drug absorption using multivariate statistics’, J. Med. Chem. 2000, 43, 3867–3877.
- 52A. K. Ghose, V. N. Viswanadhan, J. J. Wendoloski, ‘A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases’, J. Comb. Chem. 1999, 1, 55–68.
- 53I. Muegge, S. L. Heald, D. Brittelli, ‘Simple selection criteria for drug-like chemical matter’, J. Med. Chem. 2001, 44, 1841–1846.
- 54D. F. Veber, S. R. Johnson, H. Y. Cheng, B. R. Smith, K. W. Ward, K. D. Kopple, ‘Molecular properties that influence the oral bioavailability of drug candidates’, J. Med. Chem. 2002, 45, 2615–2623.
- 55J. B. Baell, G. A. Holloway, ‘New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays’, J. Med. Chem. 2010, 53, 2719–2740.
- 56G. L. Ellman, K. D. Courtney, V. Andres, R. M. Featherstone, ‘A new and rapid colorimetric determination of acetylcholinesterase activity’, Biochem. Pharmacol. 1961, 7, 88–95.
- 57D. E. Moss, ‘Improving anti-neurodegenerative benefits of acetylcholinesterase inhibitors in Alzheimer's disease: Are irreversible inhibitors the future?’ Int. J. Mol. Sci. 2020, 21, 3438.
- 58A. Daina, O. Michielin, V. Zoete, ‘SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules’, Sci. Rep. 2017, 7, 42717.
- 59P. Zakeri-Milani, H. Tajerzadeh, Z. Islambolchilar, S. Barzegar, V. Valizadeh, ‘The relation between molecular properties of drugs and their transport accross the intestinal membrane’, Daru 2006, 14, 164–171.