Antidepressant-Like Effect and Phenolic Profile of Brazilian Native and Exotic Species from Psidium Genus
Natalia Cavichioli
Laboratório de Avaliação de Substâncias Bioativas, Departamento de Ciências Naturais, Universidade Regional de Blumenau, CEP 89030-903 Blumenau, Santa Catarina, Brazil
Search for more papers by this authorAna Paula Dalmagro
Laboratório de Avaliação de Substâncias Bioativas, Departamento de Ciências Naturais, Universidade Regional de Blumenau, CEP 89030-903 Blumenau, Santa Catarina, Brazil
Search for more papers by this authorOtto Rodolfo Sasse
Laboratório de Avaliação de Substâncias Bioativas, Departamento de Ciências Naturais, Universidade Regional de Blumenau, CEP 89030-903 Blumenau, Santa Catarina, Brazil
Search for more papers by this authorLucas Henrique Junges
Laboratório de Avaliação de Substâncias Bioativas, Departamento de Ciências Naturais, Universidade Regional de Blumenau, CEP 89030-903 Blumenau, Santa Catarina, Brazil
Search for more papers by this authorAndrey Martinez Rebelo
Empresa de Pesquisa Agropecuária e Extensão Rural de Santa Catarina, EPAGRI, CEP 88318-112 Itajaí, Santa Catarina, Brazil
Search for more papers by this authorCássia Katrin Reinke
Serviço Nacional de Aprendizagem Industrial, SENAI, Instituto SENAI de Tecnologia Ambiental, CEP 89637-050 Blumenau, Santa Catarina, Brazil
Search for more papers by this authorCorresponding Author
Ana Lúcia Bertarello Zeni
Laboratório de Avaliação de Substâncias Bioativas, Departamento de Ciências Naturais, Universidade Regional de Blumenau, CEP 89030-903 Blumenau, Santa Catarina, Brazil
Programa de Pós-Graduação em Biodiversidade, Departamento de Ciências Naturais, Universidade Regional de Blumenau, CEP 89030-903 Blumenau, Santa Catarina, Brazil
Search for more papers by this authorNatalia Cavichioli
Laboratório de Avaliação de Substâncias Bioativas, Departamento de Ciências Naturais, Universidade Regional de Blumenau, CEP 89030-903 Blumenau, Santa Catarina, Brazil
Search for more papers by this authorAna Paula Dalmagro
Laboratório de Avaliação de Substâncias Bioativas, Departamento de Ciências Naturais, Universidade Regional de Blumenau, CEP 89030-903 Blumenau, Santa Catarina, Brazil
Search for more papers by this authorOtto Rodolfo Sasse
Laboratório de Avaliação de Substâncias Bioativas, Departamento de Ciências Naturais, Universidade Regional de Blumenau, CEP 89030-903 Blumenau, Santa Catarina, Brazil
Search for more papers by this authorLucas Henrique Junges
Laboratório de Avaliação de Substâncias Bioativas, Departamento de Ciências Naturais, Universidade Regional de Blumenau, CEP 89030-903 Blumenau, Santa Catarina, Brazil
Search for more papers by this authorAndrey Martinez Rebelo
Empresa de Pesquisa Agropecuária e Extensão Rural de Santa Catarina, EPAGRI, CEP 88318-112 Itajaí, Santa Catarina, Brazil
Search for more papers by this authorCássia Katrin Reinke
Serviço Nacional de Aprendizagem Industrial, SENAI, Instituto SENAI de Tecnologia Ambiental, CEP 89637-050 Blumenau, Santa Catarina, Brazil
Search for more papers by this authorCorresponding Author
Ana Lúcia Bertarello Zeni
Laboratório de Avaliação de Substâncias Bioativas, Departamento de Ciências Naturais, Universidade Regional de Blumenau, CEP 89030-903 Blumenau, Santa Catarina, Brazil
Programa de Pós-Graduação em Biodiversidade, Departamento de Ciências Naturais, Universidade Regional de Blumenau, CEP 89030-903 Blumenau, Santa Catarina, Brazil
Search for more papers by this authorAbstract
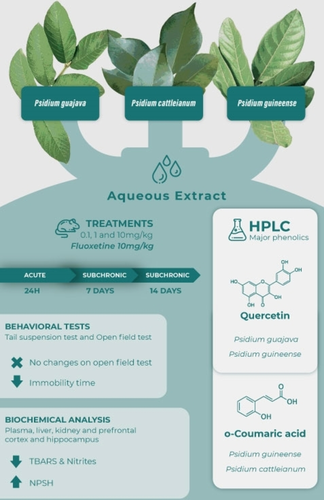
Depression is one of the disorders involving mental health that most affects the population worldwide. Considering the available pharmacological therapies for the treatment of depression are ineffective in most patients, the search for new alternatives is crucial. In line with this, we investigated the phenolic profile, antidepressant-like, and antioxidant effects triggered by the administration of aqueous extracts from Psidium guajava L. (GUA), Psidium cattleianum Sw. (CAT), and Psidium guineense Sabine (GUI) leaves in mice. Our results show that quercetin is the major compound of GUA and GUI, and o-coumaric acid in CAT extracts. The acute and subchronic administrations of the three plant extracts exerted an antidepressant-like effect in mice exposed to the tail suspension test, without changes on locomotor performance evaluated by the open field test. Furthermore, the GUI and CAT decreased oxidative stress markers, mainly lipid peroxidation and nitrites in the hippocampus, prefrontal cortex, liver, and plasma. Notably, GUA and CAT increased non-protein thiols in all tissues. Therefore, the Psidium extracts demonstrated an antidepressant-like effect in mice, and the antioxidant capacity of the extracts seems to underlie the behavioral effect.
Graphical Abstract
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1J. Martins, S. Brijesh, ‘Phytochemistry and pharmacology of anti-depressant medicinal plants: a review’, Biomed. Pharmacother. 2018, 104, 343–365.
- 2World Health Organization ‘Depression and other common mental disorders: global health estimates’ (No. WHO/MSD/MER/2017.2), Geneva, 2017.
- 3M. Maes, P. Galecki, Y. S. Chang, M. Berk, ‘A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness’, Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 676–692.
- 4A. H. Miller, C. L. Raison, ‘The role of inflammation in depression: from evolutionary imperative to modern treatment target’, Nat. Rev. Immunol. 2016, 16, 22–34.
- 5C. Otte, S. M. Gold, B. W. Penninx, C. M. Pariante, A. Etkin, M. Fava, ‘Major depressive disorder’, Nature Review Disease Primers 2016, 2, 1–20.
- 6S. Bhatt, A. N. Nagappa, C. R. Patil, ‘Role of oxidative stress in depression’, Drug Discovery Today 2020, 25, 1270–1276.
- 7O. Berton, E. J. Nestler, ‘New approaches to antidepressant drug discovery: beyond monoamines’, Nat. Rev. Neurosci. 2006, 7, 137–151.
- 8J. Mann, ‘Secondary metabolism’, Oxford University Press, London. 1978.
- 9S. M. Rates, ‘Plants as source of drugs’, Toxicon 2001, 39, 603–613.
- 10Z. J. Zhang, ‘Therapeutic effects of herbal extracts and constituents in animal models of psychiatric disorders’, Life Sci. 2004, 75, 1659–1699.
- 11H. Nasri, H. Shirzad, ‘Toxicity and safety of medicinal plants’, J. Herb. Pharmacother. 2013, 2, 21–22.
- 12A. Gupta, P. P. Singh, P. Singh, K. Singh, A. V. Singh, S. K. Singh, A. Kuma, ‘Medicinal plants under climate change: impacts on pharmaceutical properties of plants’ in ‘Climate change and agricultural ecosystems’, Digital Science & Research Solutions, Inc., 2019, pp. 181–209.
- 13J. C. Medina, ‘Goiaba: cultura, matéria prima, processamento e aspectos econômicos’, Instituto de tecnologias de alimentos. Campinas, Brazil, 1988.
- 14M. Heinrich, A. Ankli, B. Frei, C. Weimann, O. Sticher, ‘Medicinal plants in Mexico: healers’ consensus and cultural importance’, Social Science & Medicine 1998, 47, 1859–1871.
- 15A. Aguilar, A. Argueta, L. Cano, ‘Flora medicinal indígena de México: Centro y Occidente’, Instituto Nacional Indigenista, Mexico, 1994.
- 16O. Pardo, ‘Estudio comparativo de ocho especies Americanas de uso medicinal em Mozambique’, Revista Chilena de Flora y Vegetación. 1999, 1, 323–328.
- 17P. Conway, ‘Tree Medicine: A comprehensive guide to the healing power of over 170 trees’, 2001. Judy Piatkus (Publishers) Ltd, p. 2173–2177, 2002.
- 18F. B. Holetz, G. L. Pessini, N. R. Sanches, D. A. G. Cortez, C. V. Nakamura, B. P. Dias Filho, ‘Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases’, Mem. Inst. Oswaldo Cruz 2002, 97, 1027–1031.
- 19J. A. Ojewole, ‘Anti-inflammatory and analgesic effects of Psidium guajava Linn. (Myrtaceae) leaf aqueous extract in rats and mice’, Methods Find. Exp. Clin. Pharmacol. 2006, 28, 441–446.
- 20R. M. Gutiérrez, S. Mitchell, R. V. Solis, ‘Psidium guajava: A review of its traditional uses, phytochemistry and pharmacology’, J. Ethnopharmacol. 2008, 117, 1–27.
- 21E. H. Han, Y. P. Hwang, J. H. Choi, J. H. Yang, J. K. Seo, Y. C. Chung, H. G. Jeong, ‘Psidium guajava extract inhibits thymus and activation-regulated chemokine (TARC/CCL17) production in human keratinocytes by inducing heme oxygenase-1 and blocking NF-κB and STAT1 activation’, Environ. Toxicol. Pharmacol. 2011, 32, 136–145.
- 22T. Tella, B. Masola, S. Mukaratirwa, ‘The effect of Psidium guajava aqueous leaf extract on liver glycogen enzymes, hormone sensitive lipase and serum lipid profile in diabetic rats’, Biomed. Pharmacother. 2019, 109, 2441–2446.
- 23A. L. B. Zeni, A. Camargo, A. P. Dalmagro, ‘Ferulic acid reverses depression-like behavior and oxidative stress induced by chronic corticosterone treatment in mice’, Steroids 2017, 125, 131–136.
- 24J. Lenzi, A. F. Rodrigues, A. S. Rós, A. B. de Castro, D. D. de Lima, D. D. D. Magro, A. L. B. Zeni, ‘Ferulic acid chronic treatment exerts antidepressant-like effect: role of antioxidant defense system’, Metab. Brain Dis. 2015, 30, 1453–1463.
- 25A. P. Dalmagro, A. Camargo, A. L. B. Zeni, ‘Morus nigra and its major phenolic, syringic acid, have antidepressant-like and neuroprotective effects in mice’, Metab. Brain Dis. 2017, 32, 1963–1973.
- 26A. P. Dalmagro, A. Camargo, A. L. S. Rodrigues, A. L. B. Zeni, ‘Involvement of PI3 K/Akt/GSK-3β signaling pathway in the antidepressant-like and neuroprotective effects of Morus nigra and its major phenolic, syringic acid’, Chem.-Biol. Interact. 2019, 314, 108843.
- 27A. P. Dalmagro, A. Camargo, N. B. Pedron, S. A. M. Garcia, A. L. B. Zeni, ‘Morus nigra leaves extract revokes the depressive-like behavior, oxidative stress, and hippocampal damage induced by corticosterone: a pivotal role of the phenolic syringic acid’, Behav. Pharmacol. 2020, 31, 397–406.
- 28R. C. Franzon, L. D. Campos, C. E. Proença, J. C. Sousa-Silva, ‘Araçás do gênero Psidium: principais espécies, ocorrências, descrição e usos’, Brasília, DF:.Embrapa Cerrados. 2009.
- 29L. Z. O. Campos, Etnobotânica do gênero Psidium L. (Myrtaceae) no Cerrado Brasileiro, Msc Thesis, Universidade de Brasília, Brasília, 2010.
- 30V. E. G. Rodrigues, D. A. Carvalho, ‘Levantamento etnobotânico de plantas medicinais no domínio do Cerrado na região do alto Rio Grande, Minas Gerais’, Rev. Bras. Plant. Med. 2007, 9, 17–35.
- 31M. R. Ritter, G. D. Sobierajski, E. P. Schenkel, L. A. Mentz, ‘Plantas usadas como medicinais no município de Ipê, RS, Brasil’, Rev. Bras. Farmacogn. 2002, 12, 51–62.
10.1590/S0102-695X2002000200001 Google Scholar
- 32G. S. Vendruscolo, S. M. K. Rates, L. A. Mentz, ‘Chemical and pharmacologic data on medicinal plants used by the community of the Ponta Grossa neighborhood, Porto Alegre, Rio Grande do Sul, Brazil’, Rev. Bras. Farmacogn. 2005, 15, 361–372.
10.1590/S0102-695X2005000400018 Google Scholar
- 33G. S. Vendruscolo, L. A. Mentz, ‘Levantamento etnobotânico das plantas utilizadas como medicinais por moradores do bairro Ponta Grossa, Porto Alegre, Rio Grande do Sul, Brasil’, Iheringia Ser. Bot. 2006, 61, 83–103.
- 34F. Q. Alvarenga, B. C. Mota, M. N. Leite, J. M. Fonseca, D. A. Oliveira, V. de Andrade Royo, R. S. Laurentiz, ‘In vivo analgesic activity, toxicity and phytochemical screening of the hydroalcoholic extract from the leaves of Psidium cattleianum Sabine’, J. Ethnopharmacol. 2013, 150, 280–284.
- 35F. L. Brighenti, E. Gaetti-Jardim Jr, M. Danelon, G. V. Evangelista, A. C. B. Delbem, ‘Effect of Psidium cattleianum leaf extract on enamel demineralization and dental biofilm composition in situ’, Arch. Oral Biol. 2012, 57, 1034–1040.
- 36A. L. B. Zeni, T. D. Moreira, A. P. Dalmagro, A. Camargo, L. A. Bini, E. L. Simionatto, D. R. Scharf, ‘Evaluation of phenolic compounds and lipid-lowering effect of Morus nigra leaves extract’, An. Acad. Bras. Cienc. 2017, 89, 2805–2815.
- 37A. L. B. Zeni, C. A. C. D. Albuquerque, F. Gonçalves, A. Latini, C. I. Tasca, R. Podestá, C. M. Pagliosa, F. S. Duarte, T. C. M. Lima, M. Maraschin, ‘Phytochemical profile, toxicity and antioxidant activity of Aloysia gratissima (Verbenaceae)’ Quim. Nova 2013, 36, 69–73.
- 38P. A. Horn, N. B. Pedron, L. H. Junges, A. M. Rebelo, H. G. Silva Filho, A. L. B. Zeni, ‘Antioxidant profile at the different stages of craft beers production: the role of phenolic compounds’, Eur. Food Res. Technol. 2021, 247, 439–452.
- 39L. Steru, R. Chermat, B. Thierry, P. Simon, ‘The tail suspension test: a new method for screening antidepressants in mice’, Psychopharmacology 1985, 85, 367–370.
- 40A. L. Zeni, A. D. Zomkowski, T. Dal-Cim, M. Maraschin, A. L. Rodrigues, C. I. Tasca, ‘Antidepressant-like and neuroprotective effects of Aloysia gratissima: investigation of involvement of L-arginine-nitric oxide-cyclic guanosine monophosphate pathway’, J. Ethnopharmacol. 2011, 13, 864–874.
- 41M. P. Cunha, D. G. Machado, L. E. Bettio, J. C. Capra, A. L. Rodrigues, ‘Interaction of zinc with antidepressants in the tail suspension test’, Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 1913–1920.
- 42L. T. Yi, J. Li, H. C. Li, D. X. Su, X. B. Quan, X. C. He, X. H. Wang, ‘Antidepressant-like behavioral, neurochemical and neuroendocrine effects of naringenin in the mouse repeated tail suspension test’, Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 39, 175–181.
- 43H. Esterbauer, K. H. Chessman, ‘Determination of aldehydic lipid peroxidation products: MDA and hydroxymonenal’, Methods Enzymol. 1991, 186, 407–421.
- 44R. L. Levine, ‘Carbonyl assay for determination of oxidatively modified proteins’, Methods Enzymol. 1994, 233, 346–357.
- 45P. Griess, ‘Bemerkungen zu der Abhandlung der HH. Weselsky und Benedikt ‘Ueber einige Azoverbindungen’’, Ber. Dtsch. Chem. Ges. 1879, 12, 426–428.
- 46G. L. Ellman, ‘Tissue sulfhydryl groups’, Arch. Biochem. Biophys. 1959 82, 70–77.
- 47O. H. Lowry, N. J. Rosebrough, A. L. Farr, R. J. Randall, ‘Protein measurement with the Folin phenol reagent’, J. Biol. Chem. 1951, 193, 265–275.
- 48L. S. Santos, E. G. Alves Filho, P. R. V. Ribeiro, G. J. Zocolo, S. M. Silva, E. P. P. de Lucena, R. E. Alves, E. S. de Brito, ‘Chemotaxonomic evaluation of different species from the Myrtaceae family by UPLC-qToF/MS-MS coupled to supervised classification based on genus’, Biochem. Syst. Ecol. 2020, 90, 104028.
- 49P. Rinwa, A. Kumar, ‘Quercetin suppress microglial neuroinflammatory response and induce antidepressant-like effect in olfactory bulbectomized rats’, Neuroscience 2013, 255, 86–98.
- 50S. Merzoug, M. L. Toumi, A. Tahraqui, ‘Quercetin mitigates adriamycin-induced anxiety- and depression-like behaviors, immune dysfunction, and brain oxidative stress in rats’, Naunyn-Schmiedeberg′s Arch. Pharmacol. 2014, 387, 921–933.
- 51V. Mehta, A. Parashar, M. Udayabanu, ‘Quercetin prevents chronic unpredictable stress induced behavioral dysfunction in mice by alleviating hippocampal oxidative and inflammatory stress’, Physiol. Behav. 2017, 171, 69–78.
- 52C. L. Hsu, C. H. Wu, S. L. Huang, G. C. Yen, ‘Phenolic compounds rutin and o-coumaric acid ameliorate obesity induced by high-fat diet in rats’, J. Agric. Food Chem. 2009, 57, 425–431.
- 53J. F. Cryan, C. Mombereau, A. Vassout, ‘The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice’, Neurosci. Biobehav. Rev. 2005, 29, 571–625.
- 54P. Palta, L. J. Samuel, E. R. Miller III, S. L. Szanton, ‘Depression and oxidative stress: results from a meta-analysis of observational studies’, Psychosom. Med. 2014, 76, 12–9.
- 55P. Galecki, J. Szemraj, M. Bienkiewicz, K. Zboralski, E. Galecka, ‘Oxidative stress parameters after combined fluoxetine and acetylsalicylic acid therapy in depressive patients’, Hum. Psychopharmacol. 2009, 24, 277–286.
- 56K. F. Nascimento, F. M. F. Moreira, J. A. Santos, C. A. L. Kassuya, J. H. R. Croda, C. A. L. Cardoso, A. S. N. Formagio, ‘Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol’, J. Ethnopharmacol. 2018, 210, 351–358.
- 57P. S. Oliveira, V. C. Chaves, M. S. P. Soares, N. P. Bona, L. T. Mendonça, F. B. Carvalho, F. M. Stefanello, ‘Southern Brazilian native fruit shows neurochemical, metabolic and behavioral benefits in an animal model of metabolic syndrome’, Metab. Brain Dis. 2018, 33, 1551–1562.
- 58S. M. L. Vasconcelos, M. O. F. Goulart, J. B. D. F. Moura, V. Manfredini, M. D. S. Benfato, L. T. Kubota, ‘Espécies reativas de oxigênio e de nitrogênio, antioxidantes e marcadores de dano oxidativo em sangue humano: Principais métodos analíticos para sua determinação’, Quim. Nova 2007, 30, 1323–1338.
- 59I. Peric, V. Costina, A. Stanisavljevic, P. Findeisen, D. Filipovic, ‘Proteomic characterization of hippocampus of chronically socially isolated rats treated with fluoxetine: depression-like behaviour and fluoxetine mechanism of action’, Neuropharmacology 2018, 135, 268–283.
- 60M. Jayachandran, R. Vinayagam, R. R. Ambati, B. Xu, S. S. M. Chung, ‘Guava leaf extract diminishes hyperglycemia and oxidative stress, prevents β-cell death, inhibits inflammation, and regulates NF-kB signaling pathway in STZ-induced diabetic rats’, BioMed Res. Int. 2018, 1–14.
- 61A. G. Vasconcelos, A. G. N. Amorim, R. C. dos Santos, J. M. T. Souza, L. K. M. de Souza, T. S. L. Araújo, L. A. D. Nicolau, L. L. Carvalho, P. E. A. de Aquino, C. S. Martins, C. D. Ropke, P. M. G. Soares, S. A. S. Kuckelhaus, J. R. Medeiros, J. R. S. A. Leite, ‘Lycopene rich extract from red guava (Psidium guajava L.) displays anti-inflammatory and antioxidant profile by reducing suggestive hallmarks of acute inflammatory response in mice’, Food Res. Int. 2017, 99, 959–968.
- 62S. A. Gibson, Ž. Korade, R. C. Shelton, ‘Oxidative stress and glutathione response in tissue cultures from persons with major depression’, J. Psychiatr. Res. 2012, 46, 1326–1332.
- 63A. H. D. S. Paulino, A. M. F. Viana, L. D. F. Bonomo, J. F. D. C. Guerra, J. M. M. Lopes, A. C. S. Rabelo, M. E. Silva, ‘Araçá (Psidium cattleianum Sabine) ameliorates liver damage and reduces hepatic steatosis in rats fed with a high-fat diet’, J. Food Nutr. Res. 2019, 7, 132–140.
- 64H. A. Savas, H. S. Gergerlioglu, F. Armutcu, H. Herken, H. R. Yilmaz, E. Kocoglu, O. Akyol, ‘Elevated serum nitric oxide and superoxide dismutase in euthymic bipolar patients: impact of past episodes’, World J. Biol. Psychiatry 2006, 7, 51–55.
- 65N. J. Klinedinst, W. T. A. Regenold, ‘Mitochondrial bioenergetic basis of depression’, J. Bioenerg. Biomembr. 2015, 47, 155–171.
- 66E. Suzuki, G. Yagi, T. Nakaki, S. Kanba, M. Asai, ‘Elevated plasma nitrate levels in depressive states’, J. Affective Disord. 2001, 63, 221–224.
- 67M. Yanik, H. Vural, H. Tutkun, S. S. Zoroglu, H. A. Savas, H. Herken, Ö. Akyol, ‘The role of the arginine-nitric oxide pathway in the pathogenesis of bipolar affective disorder’, European Archives of Psychiatry and Clinical Neuroscience 2004, 254, 43–47.
- 68A. Harkin, T. J. Connor, M. P. Burns, J. P. Kelly, ‘Nitric oxide synthase inhibitors augment the effects of serotonin re-uptake inhibitors in the forced swimming test’, Eur. Neuropsychopharmacol. 2004, 14, 274–281.
- 69F. R. Saber, G. A. Abdelbary, M. M. Salama, D. O. Saleh, M. M. Fathy, F. M. Soliman, ‘UPL, C/QTOF/MS profiling of two Psidium species and the in vivo hepatoprotective activity of their nano-formulated liposomes’, Food Res. Int. 2018, 105, 1029–1038.
- 70E. G. Giannini, R. Testa, V. Savarino, ‘Liver enzyme alteration: a guide for clinicians’, CMAJ 2005, 172, 367–379.