Quinoline Based Monocarbonyl Curcumin Analogs as Potential Antifungal and Antioxidant Agents: Synthesis, Bioevaluation and Molecular Docking Study
Amol A. Nagargoje
Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, 431004 India
Department of Chemistry, Khopoli Municipal Council College, Khopoli, Raigad, 410203 India
Search for more papers by this authorSatish V. Akolkar
Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, 431004 India
Search for more papers by this authorMadiha M. Siddiqui
Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, 431004 India
Search for more papers by this authorDnyaneshwar D. Subhedar
Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, 431004 India
Search for more papers by this authorJaiprakash N. Sangshetti
Department of Pharmaceutical Chemistry, Y. B. Chavan College of Pharmacy, Rafiq Zakaria Campus, Aurangabad, 431001 India
Search for more papers by this authorVijay M. Khedkar
Department of Pharmaceutical Chemistry, Shri Vile Parle Kelavani Mandal's Institute of Pharmacy, Mumbai-Agra National Highway, Dhule, 424001 India
Search for more papers by this authorCorresponding Author
Bapurao B. Shingate
Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, 431004 India
Search for more papers by this authorAmol A. Nagargoje
Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, 431004 India
Department of Chemistry, Khopoli Municipal Council College, Khopoli, Raigad, 410203 India
Search for more papers by this authorSatish V. Akolkar
Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, 431004 India
Search for more papers by this authorMadiha M. Siddiqui
Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, 431004 India
Search for more papers by this authorDnyaneshwar D. Subhedar
Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, 431004 India
Search for more papers by this authorJaiprakash N. Sangshetti
Department of Pharmaceutical Chemistry, Y. B. Chavan College of Pharmacy, Rafiq Zakaria Campus, Aurangabad, 431001 India
Search for more papers by this authorVijay M. Khedkar
Department of Pharmaceutical Chemistry, Shri Vile Parle Kelavani Mandal's Institute of Pharmacy, Mumbai-Agra National Highway, Dhule, 424001 India
Search for more papers by this authorCorresponding Author
Bapurao B. Shingate
Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, 431004 India
Search for more papers by this authorAbstract
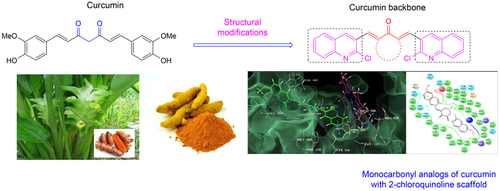
In search for new fungicidal and free radical scavenging agents, we synthesized a focused library of 2-chloroquinoline based monocarbonyl analogs of curcumin (MACs). The synthesized MACs were evaluated for in vitro antifungal and antioxidant activity. The antifungal activity was evaluated against five different fungal strains such as Candida albicans, Fusarium oxysporum, Aspergillus flavus, Aspergillus niger, and Cryptococcus neoformans, respectively. Most of the synthesized MACs displayed promising antifungal activity compared to the standard drug Miconazole. Furthermore, molecular docking study on a crucial fungal enzyme sterol 14α-demethylase (CYP51) could provide insight into the plausible mechanism of antifungal activity. MACs were also screened for in vitro radical scavenging activity using butylated hydroxytoluene (BHT) as a standard. Almost all MACs exhibited better antioxidant activity compared to BHT.
Graphical Abstract
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv201900624-sup-0001-misc_information.pdf3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1D. A. Enoch, H. A. Ludlam, N. M. Brown, ‘Invasive Fungal Infections: A Review of Epidemiology and Management Options’, J. Med. Microbiol. 2006, 55, 809–818.
- 2J. P. Latge, ‘Aspergillus fumigatus and Aspergillosis’, Clin. Microbiol. Rev. 1999, 12, 310–350.
- 3J. N. Steenbergen, A. Casadevall, ‘Prevalence of Cryptococcus neoformans var. neoformans (Serotype D) and Cryptococcus neoformans var. grubii (Serotype A) Isolates in New York City’, J. Clin. Microbiol. 2000, 38, 1974–1976.
- 4D. J. Sheehan, C. A. Hitchcock, C. M. Sibley, ‘Current and Emerging Azole Antifungal Agents’, Clin. Microbiol. Rev. 1999, 12, 40–79.
- 5I. A. Casalinuovo, P. Di Francesco, E. Garaci, ‘Fluconazole Resistance in Candida albicans: A Review of Mechanisms’, Eur. Rev. Med. Pharmacol. Sci. 2008, 8, 69–77.
- 6H. L. Hoffman, E. J. Ernst, M. E. Klepser, ‘Novel Triazole Antifungal Agents’, Expert Opin. Invest. Drugs 2000, 9, 593–605.
- 7M. A. Pfaller, ‘Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment’, Am. J. Med. Sci. 2012, 125, s 3–s13.
- 8J. M. Lü, P. H. Lin, Q. Yao, C. Chen, ‘Chemical and Molecular Mechanisms of Antioxidants: Experimental Approaches and Model Systems’, J. Cell. Mol. Med. 2010, 14, 840–860.
- 9C. R. da Silva, J. B. de Andrade Neto, J. J. C. Sidrim, M. R. F. Angelo, H. I. F. Magalhaes, B. C. Cavalcanti, R. S. N. Brilhante, D. S. Macedo, M. O. de Moraes, M. D. P. Lobo, ‘Synergistic Effects of Amiodarone and Fluconazole on Candida Tropicalis Resistant to Fluconazole’, Antimicrob. Agents Chemother. 2013, 57, 1691–1700.
- 10R. C. Reddy, P. G. Vatsala, V. G. Keshamouni, G. Padmanaban, P. N. Rangarajan, ‘Curcumin for Malaria Therapy’, Biochem. Biophys. Res. Commun. 2005, 326, 472–474.
- 11C. V. B. Martins, D. L. Da Silva, A. T. M. Neres, T. F. F. Magalhaes, G. A. Watanabe, L. V. Modolo, A. A. Sabino, A. De Fatima, M. A. De Resende, ‘Curcumin as a Promising Antifungal of Clinical Interest’, J. Antimicrob. Chemother. 2009, 63, 337–339.
- 12S. Z. Moghadamtousi, H. A. Kadir, P. Hassandarvish, H. Tajik, S. Abubakar, K. Zandi, ‘A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin’, BioMed Res. Int. 2014, 1, 1–13.
- 13A. Marchiani, C. Rozzo, A. Fadda, G. Delogu, P. Ruzza, ‘Curcumin and Curcumin-like Molecules: From Spice to Drugs’, Curr. Med. Chem. 2013, 21, 204–222.
- 14A. Goel, A. B. Kunnumakkara, B. B. Aggarwal, ‘Curcumin: From Kitchen to Clinic’, Biochem. Pharmacol. 2008, 75, 787–809.
- 15P. Anand, A. B. Kunnumakkara, R. A. Newman, B. B. Aggarwal, ‘Bioavailability of Curcumin: Problems and Promises’, Mol. Pharm. 2007, 4, 807–818.
- 16R. K. Maheshwari, A. K. Singh, J. Gaddipati, R. C. Srimal, ‘Multiple Biological Activities of Curcumin: A Short Review’, Life Sci. 2006, 78, 2081–2087.
- 17H. Hatcher, R. Planalp, J. Cho, F. M. Torti, S. V. Torti, ‘Curcumin: From Ancient Medicine to Current Clinical Trials’, Cell. Mol. Life Sci. 2008, 65, 1631–1652.
- 18R. A. Sharma, A. J. Gescher, W. P. Steward, ‘Curcumin: The Story so Far’, Eur. J. Cancer 2005, 41, 1955–1968.
- 19E. Burgos-Moron, J. M. Calderon-Montano, J. Salvador, A. Robles, M. Lopez-Lazaro, ‘The Dark Side of Curcumin’, Int. J. Cancer 2010, 126, 1771–1775.
- 20S. S. Sardjiman, M. S. Reksohadiprodjo, L. Hakim, H. Van Der Goot, H. Timmerman, ‘1,5-Diphenyl-1,4-Pentadiene-3-Ones and Cyclic Analogs as Antioxidative Agents: Synthesis and Structure−activity Relationship’, Eur. J. Med. Chem. 1997, 32, 625–630.
- 21D. Shetty, Y. J. Kim, H. Shim, J. P. Snyder, ‘Eliminating the Heart from the Curcumin Molecule: Monocarbonyl Curcumin Mimics (MACs)’, Molecules 2015, 20, 249–292.
- 22K. M. Nelson, J. L. Dahlin, J. Bisson, J. Graham, G. F. Pauli, M. A. Walters, ‘The Essential Medicinal Chemistry of Curcumin Miniperspective’, J. Med. Chem. 2017, 60, 1620–1637.
- 23T. P. Robinson, T. Ehlers, R. B. Hubbard IV, X. Bai, J. L. Arbiser, D. J. Goldsmith, J. P. Bowen, ‘Design, Synthesis, and Biological Evaluation of Angiogenesis Inhibitors: Aromatic Enone and Dienone Analogs of Curcumin’, Bioorg. Med. Chem. Lett. 2003, 13, 115–117.
- 24H. Ohtsu, Z. Xiao, J. Ishida, M. Nagai, H.-K. Wang, H. Itokawa, C.-Y. Su, C. Shih, T. Chiang, E. Chang, ‘Curcumin Analogs as Novel Androgen Receptor Antagonists with Potential as Anti-Prostate Cancer Agents’, J. Med. Chem. 2002, 45, 5037–5042.
- 25K. Bairwa, J. Grover, M. Kania, S. M. Jachak, ‘Recent Developments in Chemistry and Biology of Curcumin Analogs’, RSC Adv. 2014, 4, 13946–13978.
- 26A. Tiwari, S. Kumar, R. Shivahare, P. Kant, S. Gupta, S. N. Suryawanshi, ‘Chemotherapy of Leishmaniasis Part XIII: Design and Synthesis of Novel Heteroretinoid-Bisbenzylidine Ketone Hybrids as Antileishmanial Agents’, Bioorg. Med. Chem. Lett. 2015, 25, 410–413.
- 27S. F. P. Braga, E. V. P. Alves, R. S. Ferreira, J. R. B. Fradico, P. S. Lage, M. C. Duarte, T. G. Ribeiro, P. A. S. Junior, A. J. Romanha, M. L. Tonini, ‘Synthesis and Evaluation of the Antiparasitic Activity of Bis(Arylmethylidene) Cycloalkanones’, Eur. J. Med. Chem. 2014, 71, 282–289.
- 28P. Yu, L. Dong, Y. Zhang, W. Chen, S. Xu, Z. Wang, X. Shan, J. Zhou, Z. Liu, G. Liang, ‘Design, Synthesis and Biological Activity of Novel Asymmetric C66 Analogs as Anti-Inflammatory Agents for the Treatment of Acute Lung Injury’, Eur. J. Med. Chem. 2015, 94, 436–446.
- 29T. Kalai, M. L. Kuppusamy, M. Balog, K. Selvendiran, B. K. Rivera, P. Kuppusamy, K. Hideg, ‘Synthesis of N-Substituted 3,5-Bis(Arylidene)-4-Piperidones with High Antitumor and Antioxidant Activity’, J. Med. Chem. 2011, 54, 5414–5421.
- 30S. Y. Chen, Y. Chen, Y. P. Li, S. H. Chen, J. H. Tan, T. M. Ou, L. Q. Gu, Z. S. Huang, ‘Design, Synthesis, and Biological Evaluation of Curcumin Analogs as Multifunctional Agents for the Treatment of Alzheimer's Disease’, Bioorg. Med. Chem. 2011, 19, 5596–5604.
- 31S. Kaur, N. H. Modi, D. Panda, N. Roy, ‘Probing the Binding Site of Curcumin in Escherichia Coli and Bacillus Subtilis FtsZ - A Structural Insight to Unveil Antibacterial Activity of Curcumin’, Eur. J. Med. Chem. 2010, 45, 4209–4214.
- 32S. K. Kandi, S. Manohar, C. E. Vélez Gerena, B. Zayas, S. V. Malhotra, D. S. Rawat, ‘Curcuminoid-4-Aminoquinoline Based Molecular Hybrids: Design, Synthesis and Mechanistic Investigation of Anticancer Activity’, New J. Chem. 2015, 39, 224–234.
- 33P. R. Baldwin, A. Z. Reeves, K. R. Powell, R. J. Napier, A. I. Swimm, A. Sun, K. Giesler, B. Bommarius, T. M. Shinnick, J. P. Snyder, ‘Monocarbonyl Analogs of Curcumin Inhibit Growth of Antibiotic Sensitive and Resistant Strains of Mycobacterium Tuberculosis’, Eur. J. Med. Chem. 2015, 92, 693–699.
- 34W. S. Hamama, M. E. Ibrahim, A. A. Gooda, H. H. Zoorob, ‘Recent Advances in the Chemistry of 2-Chloroquinoline-3-Carbaldehyde and Related Analogs’, RSC Adv. 2018, 8484–8515.
- 35M. Guo, C. J. Zheng, M. X. Song, Y. Wu, L. P. Sun, Y. J. Li, Y. Liu, H. R. Piao, ‘Synthesis and Biological Evaluation of Rhodanine Derivatives Bearing a Quinoline Moiety as Potent Antimicrobial Agents’, Bioorg. Med. Chem. Lett. 2013, 23, 4358–4361.
- 36N. C. Desai, K. M. Rajpara, V. V. Joshi, ‘Synthesis and Characterization of Some New Quinoline Based Derivatives Endowed with Broad Spectrum Antimicrobial Potency’, Bioorg. Med. Chem. Lett. 2012, 22, 6871–6875.
- 37V. Ramesh, B. Ananda Rao, P. Sharma, B. Swarna, D. Thummuri, K. Srinivas, V. G. M. Naidu, V. Jayathirtha Rao, ‘Synthesis and Biological Evaluation of New Rhodanine Analogs Bearing 2-Chloroquinoline and Benzo[h]Quinoline Scaffolds as Anticancer Agents’, Eur. J. Med. Chem. 2014, 83, 569–580.
- 38A. H. Kategaonkar, P. V. Shinde, S. K. Pasale, B. B. Shingate, M. S. Shingare, ‘Synthesis and Biological Evaluation of New 2-Chloro-3-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)quinoline Derivatives via Click Chemistry Approach’, Eur. J. Med. Chem. 2010, 45, 3142–3146.
- 39S. B. Marganakop, R. R. Kamble, T. Taj, M. Y. Kariduraganvar, ‘An Efficient One-Pot Cyclization of Quinoline Thiosemicarbazones to Quinolines Derivatized with 1,3,4-Thiadiazole as Anticancer and Anti-Tubercular Agents’, Med. Chem. Res. 2012, 21, 185–191.
- 40T. Aravinda, H. S. Bhojya Naik, H. R. Prakash Naik, ‘1,2,3-Triazole Fused Quinoline-Peptidomimetics: Studies on Synthesis, DNA Binding and Photonuclease Activity’, Int. J. Pept. Res. Ther. 2009, 15, 273–279.
- 41D. D. Subhedar, M. H. Shaikh, L. Nawale, D. Sarkar, V. M. Khedkar, B. B. Shingate, ‘Quinolidene Based Monocarbonyl Curcumin Analogs as Promising Antimycobacterial Agents: Synthesis and Molecular Docking Study’, Bioorg. Med. Chem. Lett. 2017, 27, 922–928.
- 42A. A. Nagargoje, S. V. Akolkar, M. M. Siddiqui, A. V. Bagade, K. M. Kodam, J. N. Sangshetti, M. G. Damale, B. B. Shingate, ‘Synthesis and Evaluation of Pyrazole-Incorporated Monocarbonyl Curcumin Analogs as Antiproliferative and Antioxidant Agents’, J. Chin. Chem. Soc. 2019, 66, 1658–1665.
- 43T. R. Deshmukh, V. S. Krishna, D. Sriram, J. N. Sangshetti, B. B. Shingate, ‘Synthesis and Bioevaluation of α,α′-Bis(1H-1,2,3-triazol-5-ylmethylene) Ketones’, Chem. Papers 2019, doi: 10.1007/s11696-019-00908-5.
- 44B. Duraiswamy, S. Mishra, V. Subhashini, S. Dhanraj, B. Suresh, ‘Studies on the Antimicrobial Potential of Mahonia leschenaultii Takeda Root and Root Bark’, Indian J. Pharm. Sci. 2009, 68, 389.
10.4103/0250-474X.26671 Google Scholar
- 45K. L. Therese, R. Bagyalakshmi, H. N. Madhavan, P. Deepa, ‘In–Vitro Susceptibility Testing by Agar Dilution Method to Determine the Minimum Inhibitory Concentrations of Amphotericin B, Fluconazole and Ketoconazole against Ocular Fungal Isolates’, Indian J. Med. Microbiol. 2006, 24, 273–279.
- 46C. H. Collines, ‘Microbiological Methods’, Butterworth-Heinemann, London, 1967.
- 47M. Burits, F. Bucar, ‘Antioxidant Activity of Nigella sativa Essential Oil’, Phytother. Res. 2000, 14, 323–328.
- 48R. A. Friesner, R. B. Murphy, M. P. Repasky, L. L. Frye, J. R. Greenwood, T. A. Halgren, P. C. Sanschagrin, D. T. Mainz, ‘Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein-Ligand Complexes’, J. Med. Chem. 2006, 49, 6177–6196.
- 49T. A. Halgren, R. B. Murphy, R. A. Friesner, H. S. Beard, L. L. Frye, W. T. Pollard, J. L. Banks, ‘Glide: A New Approach for Rapid, Accurate Docking and Scoring. 2. Enrichment Factors in Database Screening’, J. Med. Chem. 2004, 47, 1750–1759.